Volume 23, Number 6—June 2017
Research
Distribution and Quantitative Estimates of Variant Creutzfeldt-Jakob Disease Prions in Tissues of Clinical and Asymptomatic Patients
On This Page
Jean Y. Douet, Caroline Lacroux, Naima Aron, Mark W. Head, Séverine Lugan, Cécile Tillier, Alvina Huor, Hervé Cassard, Mark Arnold, Vincent Beringue, James W. Ironside, and Olivier Andréoletti
Abstract
In the United-Kingdom, ≈1 of 2,000 persons could be infected with variant Creutzfeldt-Jakob disease (vCJD). Therefore, risk of transmission of vCJD by medical procedures remains a major concern for public health authorities. In this study, we used in vitro amplification of prions by protein misfolding cyclic amplification (PMCA) to estimate distribution and level of the vCJD agent in 21 tissues from 4 patients who died of clinical vCJD and from 1 asymptomatic person with vCJD. PMCA identified major levels of vCJD prions in a range of tissues, including liver, salivary gland, kidney, lung, and bone marrow. Bioassays confirmed that the quantitative estimate of levels of vCJD prion accumulation provided by PMCA are indicative of vCJD infectivity levels in tissues. Findings provide critical data for the design of measures to minimize risk for iatrogenic transmission of vCJD.
Prion diseases, or transmissible spongiform encephalopathies (TSEs), are fatal neurodegenerative disorders that occur naturally in sheep (scrapie), cattle (bovine spongiform encephalopathy [BSE]), and humans (Creutzfeldt-Jakob disease [CJD]). A key event in the pathogenesis of TSEs is the conversion of the normal cellular prion protein (PrPC, encoded by the PRNP gene) into an abnormal disease-associated isoform (PrPSc) in tissues of infected animals. PrPC is completely degraded after controlled digestion with proteinase K in the presence of nondenaturing detergents. In contrast, PrPSc is N terminally truncated under the same conditions, resulting in a proteinase K–resistant prion (PrPres) (1).
In 1996, a new form of CJD, termed variant CJD (vCJD) was identified in the United Kingdom. vCJD is believed to result from zoonotic transmission of the BSE agent, probably as a consequence of dietary exposure to BSE-contaminated meat products (2,3). The total number of clinical cases of vCJD thus far identified is limited (227 patients worldwide at the time of writing this article). However, the estimated prevalence of asymptomatic vCJD in populations exposed to the BSE agent is uncertain (4).
In the United Kingdom, 32,441 appendix samples collected during surgery from patients born during 1941–1985 have been tested for abnormal prion protein accumulation by using immunohistochemical analysis. This study reported a vCJD prevalence estimate of 1/2,000 in persons in these age cohorts (95% CI 1/3,500–1/1,250) (5). No comparable data are available concerning the prevalence of asymptomatic vCJD in other countries, although BSE exposure is known to have occurred in several countries in continental Europe, as judged by cases of vCJD that are not attributable to exposure in the United Kingdom (http://www.cjd.ed.ac.uk/documents/worldfigs.pdf).
Over the past 2 decades, several studies have reported on the distribution of the vCJD agent in tissues of infected patients (6–8). Most of these studies did not detect the vCJD agent outside the nervous system (central, peripheral, and autonomic) and lymphoid tissues. However, the sensitivity of detection techniques for PrPres used in these investigations was limited.
Protein misfolding cyclic amplification (PMCA) is believed to mimic prion replication in vitro, but in an accelerated form, which enables amplification of minute amounts of PrPSc and prion infectivity (9). In PMCA, a PrPC-containing substrate is combined with a seed that might contain otherwise undetectable amounts of PrPSc. After repeated cycles of incubation and sonication, the amount of PrPSc increases to levels at which they can be detected by using conventional biochemical techniques. Recently, our group and others have shown that PMCA can detect endogenous vCJD agent in patient biologic fluids such as urine and blood (10,11).
In this study, we evaluated the relative sensitivity of PMCA versus that of bioassay in mice for detection of the vCJD agent. We estimated by using PMCA the level of vCJD prions in 21 tissues collected from 4 patients who died of symptomatic vCJD and from a patient with asymptomatic vCJD. We also determined whether vCJD prion levels, as estimated by using PMCA, were consistent with infectious titers, as estimated by bioassay with transgenic mice.
Ethics Statements
All animal experiments were performed in compliance with institutional and French national guidelines and in accordance with the European Community Council Directive 86/609/EEC. Animal experiments that were part of this study (national registration no. 01734.01) were approved by the local ethic committee of the Ecole Nationale Vétérinaire de Toulouse (Toulouse, France). Mouse inoculations were performed under anesthesia with isofulorane. Mice that displayed clinical signs of disease were anesthetized with isofluorane before being humanely killed by inhalation of CO2.
Human samples were obtained from the United Kingdom National CJD Research and Surveillance Unit Brain and Tissue Bank, which is part of the Medical Research Council Edinburgh Brain Bank (Edinburgh, Scotland, UK). Tissue samples were pseudo-anonymized by using a Brain Bank reference number. All case-patients in the United Kingdom provided informed consent. Use of samples in this study was approved by the East of Scotland Research Ethics Service for the Edinburgh Brain Bank (16/ES/0084).
vCJD and Control Patients
We investigated tissues from 4 clinical vCJD case-patients (vCJD-1–vCJD-4) and 1 asymptomatic person with vCJD who had received a transfusion of packed erythrocytes from a donor who subsequently died from vCJD (12). Tissues from 2 non–vCJD–affected patients were used as controls. For case-patients who provided appropriate consent, the entire PRNP gene coding sequence was established to exclude pathogenic mutations in this gene (13,14).
Mouse Bioassays
Bioassays were performed by using mice expressing bovine PrP (tgBov-tg110) as described (15,16). These mice were observed daily and their neurologic status was assessed weekly. When clinically progressive TSE symptoms were evident, or at the end of their lifespan, the animals were euthanized. Survival time was expressed as the mean ± SD days postinoculation of mice positive for PrPres. For mice that showed no clinical signs, they were humanely killed at the end of their natural lifespan (600–800 days). In these instances, incubation periods are reported as >600 days postinoculation, which corresponded to survival time observed for >3 of 6 mice.
Estimation of Infectious Prion Titers
We estimated infectious titer in a reference 10% (wt/vol) frontal cortex homogenate from a clinical vCJD patient by using endpoint titration (intracerebral route) in tgBov mice. Infectious titer (50% lethal dose/g intracerebral in tgBov mice) was estimated by using the Spearman method.
The titer of prion infectivity in vCJD–affected patient bone marrow samples was estimated by using the method of Arnold et al. (17). This method uses the probability of survival (attack rate at each dilution) and the individual mouse incubation periods at each dilution to estimate infectious load and is thus able to provide more accurate estimation of titer than using either attack rate or incubation period data alone.
PMCA Reactions
A transgenic mouse line that expresses ovine A136R154Q171 PrP variant PrPC (tgShXI) was used to prepare the PMCA substrate as described (18,19). PMCA amplification was performed as described (11). Each PMCA experiment included a reference vCJD sample (10% brain homogenate) as a control for the amplification efficiency. Unseeded controls (1 unseeded control for 8 seeded reactions) were also included in each experiment. For each tested dilution of each sample, >4 replicates were tested in 2 independent experiments. For each sample, the highest dilution showing >50% of positive replicates (presence of detectable PrPres in the reaction as assessed by using Western blotting) was determined.
Detection of Abnormal PrP by Western Blotting and Paraffin-Embedded Tissue Blotting
Extraction of proteinase K–resistant abnormal PrP and Western blotting were performed as described (11). Immunodetection was performed by using 2 PrP-specific monoclonal antibodies, Sha31 (1 μg/mL) (20), and 12B2 (4 μg/mL) (21), which recognize amino acid sequences YEDRYYRE (145–152), and WGQGG (89–93), respectively. Paraffin-embedded tissue blotting was performed as described (22,23).
Sensitivity of vCJD Agent Detection by PMCA and Bioassay
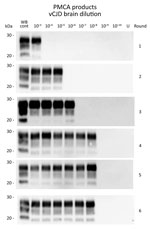
Figure 1. Western blots of variant Creutzfeldt-Jakob disease (vJCD) proteinase K–resistant prions (PrPres) analyzed by protein misfolding cyclic amplification (PMCA) in tissues of clinical and asymptomatic patients. PMCAs were seeded with a 10-fold...
To determine the relative sensitivity of PMCA, we retitrated a reference sample (10% cerebral cortex homogenate from a vCJD-affected patient) that had previously undergone endpoint titration (IC inoculation route; Table 1) in bovine PrP–expressing mice (tgBov). Amplification of a 10-fold serial dilution of this sample (6 individual replicates/dilution point) demonstrated that 4 PMCA rounds (24 hours/round, i.e., 96 h) were sufficient to reach the maximum sensitivity level of the assay. Additional PMCA rounds did not improve the analytical sensitivity of the assay or the number of positive replicates (Table 2; Figure 1). On the basis of these results, we estimated by using the Spearman method that the seeding activity of the isolate was 1011 50% seeding activity/per g. Bioassay endpoint titration data for the same sample in tgBov mice showed an infectious titer of 107.7 LD50/g. When we took into account the 4-fold lower amount of material used to seed the PMCA reaction compared with material used in mouse inoculations, we found that the PMCA protocol used was 465 times more sensitive than the bioassay of tgBov mice for detection of vCJD prions.
PMCA for Control and vCJD Patients

Figure 2. Protein misfolding cyclic amplification (PMCA) of peripheral tissues from patients with variant Creutzfeldt-Jakob disease (vCJD). PMCA reactions were seeded with a 10-fold dilution series of vCJD tissue homogenates (10−2–10−9) obtained postmortem...
We complied basic demographic data for vCJD and control patients (Table 3). A 10-fold dilution series of 10% homogenates from the vCJD–affected and non–vCJD–affected control patients was prepared, and this series was subjected to 4 rounds of PMCA. Amplification products from each round were tested for PrPres using by Western blotting (Table 4; Figure 2).
We found that none of the reactions seeded with tissue homogenates from non–CJD controls were positive for PrPres (Table 4). In contrast, PMCA reactions seeded with tissues from the 4 symptomatic vCJD patients were positive for PrPres (Table 4; Figure 2). As expected, among tested tissues, brain homogenates (temporal cortex) showed the highest seeding activity (highest PrPres-positive dilution 10−8). All lymphoid organs tested also showed seeding activity, but the highest PMCA-positive dilution varied according to the organs tested from 10−2(thymus) to 10−6 (distal ileum and tonsil). Moreover, for a given lymphoid organ, <102-fold differences was observed in seeding activity, depending on the patient and sample tested. These data indicate that for symptomatic vCJD patients, lymphoid organs contain 102–106-fold less prion seeding activity than the same amount of brain tissue (Table 4).
![Thumbnail of Western blots of proteinase K–resistant prions (PrPres) in PMCA reactions seeded with peripheral tissues. PMCA reactions were seeded with a 10-fold dilution series (10−2–10−9) of variant Creutzfeldt-Jakob disease (vCJD) tissue homogenates that had been collected postmortem from vCJD patients during the clinical stage (symptomatic vCJD patient 1–vCJD patient 4 [P1–P4]) or at an asymptomatic or preclinical stage of the disease (vCJD asymp) (Table 2). Reactions seeded with tissues from](https://wwwnc.cdc.gov/eid/images/16-1734-F3-tn.jpg)
Figure 3. Western blots of proteinase K–resistant prions (PrPres) in PMCA reactions seeded with peripheral tissues. PMCA reactions were seeded with a 10-fold dilution series (10−2–10−9) of variant Creutzfeldt-Jakob disease (vCJD) tissue homogenates...
Salivary gland, adrenal gland, liver, and bone marrow from the 4 symptomatic vCJD patients showed positive reactions by PMCA (Figures 2, 3). Using the highest dilution to show a positive reaction as a measure of seeding activity, we found that the vCJD agent in these tissues was 103–106-fold lower than that for the brain. PrPres was also detected by PMCA reactions seeded with heart, liver, kidney, skeletal muscle, several endocrine/exocrine glands (pancreas, thyroid), and gonads, from some, but not all, of the 4 clinical vCJD patients. Positive tissues contained a level of vCJD seeding activity that was equivalent to those observed in distal ileum (i.e., 103–106-fold lower than for the brain). Irrespective of the tissue used to seed the PMCA reactions, the PrPres Western blot profile for positive reactions was indistinguishable from that observed in reactions seeded with the vCJD brain control (Figure 3).
Analysis of Tissues from an Asymptomatic vCJD-Infected Person
Prion seeding activity was not detected in the brain (temporal cortex) of the asymptomatic vCJD–affected patient, who was infected with a PRNP gene codon 129 heterozygote (Met/Val129) prion (12) (Table 4; Figure 2). PMCA reactions seeded with dorsal root ganglia or trigeminal ganglia homogenates from this patient showed negative results. However, seeding activity was detected in the pituitary gland (highest PrPres-positive dilution 10−2). In addition, as for the symptomatic vCJD patient, PMCA amplification readily detected vCJD prions in all lymphoid organs tested from this asymptomatic person. On the basis of PMCA results, the vCJD agent load in lymphoid organs in this asymptomatic patient infected with the PRNP gene codon 129 Met/Val129 prion was similar to those for patients infected with Met/Met129 prions during the clinical stage of disease.
In addition to lymphoid organs, prion seeding activity was detectable in certain peripheral tissues (salivary gland, lung, and liver) from this patient (Tables 4; Figures 2, 3). Certain tissues, such as bone marrow or adrenal gland, that contained a substantial prion seeding activity in the clinically affected patients showed negative results. Again, the PrPres Western blot profile for positive reactions was indistinguishable from that observed for reactions seeded with the vCJD brain control.
vCJD Infectivity in Bone Marrow
![Thumbnail of Detection of proteinase K–resistant prions (PrPres) by using Western blotting and paraffin-embedded tissue (PET) blotting of brains of transgenic mice expressing bovine PrP (tgBov). A) PrPres WB of a vCJD sample (frontal cortex), tgBov mice (brain) inoculated with the same vCJD reference isolate, bone marrow samples from vCJD-affected patients (vCJD 1–vCJD-4 [P1–P4]; Table 2), and a non–vCJD control (NC-1; Table 2). A scrapie isolate (WB cont) and a noninoculated tgBov brain (vCJD b](https://wwwnc.cdc.gov/eid/images/16-1734-F4-tn.jpg)
Figure 4. Detection of proteinase K–resistant prions (PrPres) by using Western blotting and paraffin-embedded tissue (PET) blotting of brains of transgenic mice expressing bovine PrP (tgBov). A) PrPres WB of a vCJD sample...
To test whether PMCA seeding activity in peripheral tissues from vCJD patients correlated with infectivity, we inoculated bone marrow samples from the symptomatic patient into tgBov mice. Clinical TSE was observed in mice that were inoculated with each of the 4 bone marrow samples. The PrPres Western blot profile and the PrPres distribution pattern, as assessed by paraffin-embedded tissue blotting for brain of the bone marrow–inoculated mice, were identical to those observed in tgBov mice inoculated with the vCJD brain control sample (Figure 4).
![Thumbnail of Dose–response relationship for A) incubation period and B) probability of infection of bovine PrP–expressing mice. Data were derived from an endpoint titration of 10% (wt/vol) frontal cortex homogenate from a patient with variant Creutzfeldt-Jakob disease. This homogenate was inoculated into tgBov mice (20 μL by intracerebral [ic] route; Table 1). This procedure was used to establish a model that estimates infectious titer in a homogenate on the basis of incubation period and the pr](https://wwwnc.cdc.gov/eid/images/16-1734-F5-tn.jpg)
Figure 5. Dose–response relationship for A) incubation period and B) probability of infection of bovine PrP–expressing mice. Data were derived from an endpoint titration of 10% (wt/vol) frontal cortex homogenate from a patient...
Data obtained for mice inoculated with bone marrow samples were also used to estimate prion infectivity levels in these samples. For this purpose, we applied the method of Arnold et al. (17). This method combines the probability of survival (attack rate) and the individual mouse incubation period to provide an estimation of infectious titers. We used data corresponding to endpoint titration in tgBov mice for reference vCJD sample (frontal cortex from a clinical vCJD patient) (Table 1) to derive the relationship between prion titer of inoculum and the probability of infection and length of the incubation period (Figure 5). We found that bone marrow samples had an infectious titer that ranged from 102.3 LD50/g through 104.7 LD50/g in tgBov mice (Table 5).
These values are consistent with a 103–105 lower infectivity load in bone marrow samples than in the reference vCJD brain sample. Consistent with the PMCA results (Table 4), we found that prion load in bone marrow samples (highest PrPres–positive dilution [10−3–10−5]) was also 103–105-fold lower than for the reference vCJD isolate (highest PrPres–positive dilution [10−8]). These results strongly support the idea that PMCA seeding activity provides a reliable estimate of the prion load in tissues from vCJD-infected patients.
Most previous studies with tissue from vCJD patients have failed to identify consistent accumulation of the vCJD agent outside the nervous and lymphoreticular systems. However, data obtained in this study clearly demonstrate the presence of vCJD prions in a wide and unexpected variety of peripheral tissues.
Natural scrapie and experimental BSE in sheep are 2 models of orally transmitted prion diseases (24,25). In both diseases, the agent accumulates in the lymphoreticular system and the enteric nervous system during the early preclinical phase of the incubation period. Moreover, an early and persistent prionemia is observed in asymptomatic infected animals (26,27). These features were also observed in vCJD in humans and in view of the likely origin of vCJD (oral exposure to BSE agent), these similarities have led to a consensus that BSE and scrapie in sheep and vCJD in human have a common pathogenesis (28).
Although vCJD prions in a variety tissues, such as bone marrow, kidney, salivary gland, skeletal muscle, pancreas, liver, or heart, might be surprising, each of these tissue has already been demonstrated to accumulate prion infectivity or abnormal prion protein in TSE-infected sheep (29–33). Because low levels of infectivity have been reported in blood fractions from a vCJD-affected patient, such widespread tissue positivity might be derived from residual blood, rather than from the solid tissue in these samples (16). However, this proposal seems unlikely because in whole blood PMCA amplification inhibitors preclude detection of endogenous vCJD agent by this method (11,34–36).
The patient in our study who was infected with a prion containing PRNP gene codon 129 Met/Val is 1 of only 2 identified vCJD agent–infected persons known to have died of other causes before onset clinical symptoms of vCJD, and the only person who provided consent to sample autopsy tissues for research. For this patient, all previous investigations did not detect abnormal prion protein or infectivity in the brain (12,37). The negative PMCA results we obtained for cerebral cortex, dorsal root ganglia, and trigeminal ganglia tissue from this patient are consistent with a lack of central nervous system involvement at the time of death. However, PMCA seeding activity in the pituitary gland was surprising in this instance.
The presence of abnormal prion protein accumulation in the pituitary gland and other circumventricular organs before deposition of PrPres in surrounding brain has been reported in TSE-infected sheep (38). However, this phenomenon in animals does not represent the main route for neuroinvasion and is a probable consequence of hematogenous dissemination of the TSE agent through the fenestrated capillary system of the circumventricular organs, which is substantially more permeable than the other capillaries in the brain (blood–brain barrier). Therefore, this finding might be a consequence of the hematogenous route of secondary vCJD in this person (by transfusion of packed erythrocytes from a vCJD-infected donor), in contrast to the oral route of infection in primary clinical vCJD cases (12).
vCJD prions were detected in certain peripheral tissues from the patients infected with a prion containing the PRNP gene codon 129 Met/Val. Although distribution of vCJD seeding activity in lymphoreticular tissues was similar to that observed for symptomatic vCJD patients, several tissues that were positive in clinically affected patients were negative in this heterozygous asymptomatic person. These findings suggest that involvement of some peripheral tissues might occur at a later stage in the incubation period than others, or that they could involve recirculation of the agent from the central nervous system (i.e., centrifugal spread in a late state). However, we cannot discount the possibility that that these differences in tissue distribution are caused by the hematogenous route of infection in this person (as opposed to the probable oral route in patients with clinical vCJD) or the difference between the PRNP gene codon 129 genotype of the asymptomatic vCJD–affected person (PRNP gene codon 129 Met/Val) and persons with clinical vCJD (PRNP gene codon 129 Met/Met).
Irrespective of the actual explanation for these differences, the presence of vCJD agent in peripheral tissues of patients during preclinical and clinical stage of the disease indicates the potential for iatrogenic transmission of this fatal neurologic condition by surgical procedures. Furthermore, this finding shows that, for certain peripheral tissues, a level of infectivity equivalent to an end stage titer (and attendant risk) is reached at a preclinical stage.
Several hundred cases of iatrogenic CJD have been reported worldwide. These cases appear to result from transmission of sporadic CJD, and most cases have occurred in recipients of human dura mater grafts or after administration of human growth hormone extracted from cadaveric pituitaries (39). Although in sporadic CJD the distribution of the agent is largely restricted to the nervous system (central and peripheral), the wide distribution of the vCJD agent in the asymptomatic infected patient we report might serve to increase the range of medical procedures, including dentistry, organ transplant, and surgery involving nondisposable equipment, that might result in iatrogenic transmission of vCJD (40–43).
Nevertheless, >20 years after identification of the first vCJD patients, only 5 cases that are a probable consequence of iatrogenic vCJD transmission are known, all in the United Kingdom and associated with blood and blood products. These cases were caused by transfusion of non–leukocyte-depleted erythrocyte concentrates or by treatment involving large amounts of pooled plasma from the United Kingdom that were known to include donations from persons who later showed development of vCJD (12,44–46).
None of the 220 other vCJD cases identified worldwide have been linked to any other medical or dental procedure. Whereas this fact is reassuring, it would be unwise to disregard the threat that vCJD still poses for public health. Despite the relatively low number (n = 178) of vCJD clinical cases observed in the United Kingdom, the most recent epidemiologic studies indicate that ≈1 of 2,000 persons in the United Kingdom could be infected with the vCJD agent (as indicated by the presence of abnormal prion protein detected by immunohistochemical analysis of lymphoid follicles in the appendix). Each asymptomatic vCJD-infected person represents a potential source of secondary infection. The data in our report offer an opportunity for refining measures that were implemented in many countries to limit the risk for vCJD iatrogenic transmission. The apparent concordance between PMCA biochemical and infectivity bioassay data, and the higher analytical sensitivity of PMCA, suggest that future research need not rely exclusively on time-consuming and costly animal bioassay.
Our results indicate the need for vCJD screening assays. After more than a decade of effort, several vCJD blood detection tests have reached a stage in their development that could enable their evaluation as screening or confirmatory assays (11,47,48). In particular, there is now a strong case for use of PMCA in a highly sensitive and specific blood test for vCJD, as indicated by our previous studies (11,16) and studies by Bougard et al. (35) and Concha-Marambio et al. (36). The relationship shown here between PrPres amplification by PMCA and detection of infectivity by bioassay indicates that PMCA seeding activity is a good surrogate marker of infectivity and could provide a sound basis for a vCJD blood test for use with blood or tissue donors.
Dr. Douet is a research scientist and assistant lecturer in ophthalmology at the National Veterinary School of Toulouse, Toulouse, France. His primary research interests are the pathogenesis of the prion disease with special emphasis on the iatrogenic risk of transmission.
Acknowledgment
This study was supported in part by the Department of Health Policy Research Programme and the Scottish Government. The National CJD Research and Surveillance Unit is supported by the Policy Research Program of the Department of Health and the Scottish Government (DH121/5061). The Edinburgh Brain Bank is supported by the Medical Research Council (MRC grant G0900580). The Unité Mixte de Recherche 1225, Ecole Nationale Vétérinaire de Toulouse was supported by the European Union FEDER/INTERREG (EFA282/13 TRANSPRION), the Institut National de la Recherche Agronomique Institut Carnot en Santé Animale, and an Agence Nationale Recherche grant (Unmasking Blood Prions; ANR-15-CE18-0028).
References
- McKinley MP, Bolton DC, Prusiner SB. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983;35:57–62. DOIPubMed
- Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature. 1997;389:498–501. DOIPubMed
- Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature. 1996;383:685–90. DOIPubMed
- Garske T, Ghani AC. Uncertainty in the tail of the variant Creutzfeldt-Jakob disease epidemic in the UK. PLoS One. 2010;5:e15626. DOIPubMed
- Gill ON, Spencer Y, Richard-Loendt A, Kelly C, Dabaghian R, Boyes L, et al. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ. 2013;347(oct15 5):f5675. DOIPubMed
- Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, et al. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358:171–80. DOIPubMed
- Haïk S, Faucheux BA, Sazdovitch V, Privat N, Kemeny JL, Perret-Liaudet A, et al. The sympathetic nervous system is involved in variant Creutzfeldt-Jakob disease. Nat Med. 2003;9:1121–3. DOIPubMed
- Head MW, Ritchie D, Smith N, McLoughlin V, Nailon W, Samad S, et al. Peripheral tissue involvement in sporadic, iatrogenic, and variant Creutzfeldt-Jakob disease: an immunohistochemical, quantitative, and biochemical study. Am J Pathol. 2004;164:143–53. DOIPubMed
- Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–3. DOIPubMed
- Moda F, Gambetti P, Notari S, Concha-Marambio L, Catania M, Park KW, et al. Prions in the urine of patients with variant Creutzfeldt-Jakob disease.N Engl J Med. 2014;371:530–9. DOIPubMed
- Lacroux C, Comoy E, Moudjou M, Perret-Liaudet A, Lugan S, Litaise C, et al. Preclinical detection of variant CJD and BSE prions in blood. PLoS Pathog. 2014;10:e1004202. DOIPubMed
- Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;364:527–9. DOIPubMed
- Uro-Coste E, Cassard H, Simon S, Lugan S, Bilheude JM, Perret-Liaudet A, et al. Beyond PrPres type 1/type 2 dichotomy in Creutzfeldt-Jakob disease.PLoS Pathog. 2008;4:e1000029. DOI
- Moreno CR, Moazami-Goudarzi K, Laurent P, Cazeau G, Andreoletti O, Chadi S, et al. Which PrP haplotypes in a French sheep population are the most susceptible to atypical scrapie? Arch Virol. 2007;152:1229–32. DOIPubMed
- Castilla J, Gutiérrez Adán A, Brun A, Pintado B, Ramírez MA, Parra B, et al. Early detection of PrPres in BSE-infected bovine PrP transgenic mice. Arch Virol. 2003;148:677–91. DOIPubMed
- Douet JY, Zafar S, Perret-Liaudet A, Lacroux C, Lugan S, Aron N, et al. Detection of infectivity in blood of persons with variant and sporadic Creutzfeldt-Jakob disease. Emerg Infect Dis. 2014;20:114–7. DOIPubMed
- Arnold ME, Hawkins SA, Green R, Dexter I, Wells GA. Pathogenesis of experimental bovine spongiform encephalopathy (BSE): estimation of tissue infectivity according to incubation period. Vet Res. 2009;40:8. DOIPubMed
- Groschup MH, Buschmann A. Rodent models for prion diseases. Vet Res. 2008;39:32. DOIPubMed
- Douet JY, Lacroux C, Corbière F, Litaise C, Simmons H, Lugan S, et al. PrP expression level and sensitivity to prion infection. J Virol. 2014;88:5870–2. DOIPubMed
- Féraudet C, Morel N, Simon S, Volland H, Frobert Y, Créminon C, et al. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J Biol Chem. 2005;280:11247–58. DOIPubMed
- Langeveld JP, Jacobs JG, Erkens JH, Bossers A, van Zijderveld FG, van Keulen LJ. Rapid and discriminatory diagnosis of scrapie and BSE in retro-pharyngeal lymph nodes of sheep. BMC Vet Res. 2006;2:19. DOIPubMed
- Cassard H, Torres JM, Lacroux C, Douet JY, Benestad SL, Lantier F, et al. Evidence for zoonotic potential of ovine scrapie prions. Nat Commun. 2014;5:5821. DOIPubMed
- Lacroux C, Corbière F, Tabouret G, Lugan S, Costes P, Mathey J, et al. Dynamics and genetics of PrPSc placental accumulation in sheep. J Gen Virol. 2007;88:1056–61. DOIPubMed
- Andréoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, et al. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol. 2000;81:3115–26. DOIPubMed
- Foster JD, Parnham DW, Hunter N, Bruce M. Distribution of the prion protein in sheep terminally affected with BSE following experimental oral transmission. J Gen Virol. 2001;82:2319–26. DOIPubMed
- Lacroux C, Vilette D, Fernández-Borges N, Litaise C, Lugan S, Morel N, et al. Prionemia and leukocyte-platelet-associated infectivity in sheep transmissible spongiform encephalopathy models. J Virol. 2012;86:2056–66. DOIPubMed
- Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. Transmission of BSE by blood transfusion in sheep. Lancet. 2000;356:999–1000. DOIPubMed
- Hilton DA. Pathogenesis and prevalence of variant Creutzfeldt-Jakob disease. J Pathol. 2006;208:134–41. DOIPubMed
- Tamgüney G, Richt JA, Hamir AN, Greenlee JJ, Miller MW, Wolfe LL, et al. Salivary prions in sheep and deer. Prion. 2012;6:52–61. DOIPubMed
- Andréoletti O, Simon S, Lacroux C, Morel N, Tabouret G, Chabert A, et al. PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat Med. 2004;10:591–3. DOIPubMed
- Chianini F, Cosseddu GM, Steele P, Hamilton S, Hawthorn J, Síso S, et al. Correlation between infectivity and disease associated prion protein in the nervous system and selected edible tissues of naturally affected scrapie sheep. PLoS One. 2015;10:e0122785. DOIPubMed
- Hadlow WJ, Kennedy RC, Race RE. Natural infection of Suffolk sheep with scrapie virus. J Infect Dis. 1982;146:657–64. DOIPubMed
- Garza MC, Monzón M, Marín B, Badiola JJ, Monleón E. Distribution of peripheral PrP(Sc) in sheep with naturally acquired scrapie. PLoS One. 2014;9:e97768. DOIPubMed
- Castilla J, Saá P, Soto C. Detection of prions in blood. Nat Med. 2005;11:982–5.PubMed
- Bougard D, Brandel JP, Bélondrade M, Béringue V, Segarra C, Fleury H, et al. Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Sci Transl Med. 2016;8:370ra182. DOIPubMed
- Concha-Marambio L, Pritzkow S, Moda F, Tagliavini F, Ironside JW, Schulz PE, et al. Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci Transl Med. 2016;8:370ra183. DOIPubMed
- Bishop MT, Diack AB, Ritchie DL, Ironside JW, Will RG, Manson JC. Prion infectivity in the spleen of a PRNP heterozygous individual with subclinical variant Creutzfeldt-Jakob disease. Brain. 2013;136:1139–45. DOIPubMed
- Sisó S, González L, Jeffrey M. Neuroinvasion in prion diseases: the roles of ascending neural infection and blood dissemination. Interdiscip Perspect Infect Dis. 2010;2010:747892.
- Brown P, Brandel JP, Sato T, Nakamura Y, MacKenzie J, Will RG, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901–7. DOIPubMed
- Flechsig E, Hegyi I, Enari M, Schwarz P, Collinge J, Weissmann C. Transmission of scrapie by steel-surface-bound prions. Mol Med. 2001;7:679–84.PubMed
- Taylor D. Inactivation of the BSE agent. C R Biol. 2002;325:75–6. DOIPubMed
- Molesworth A, Yates P, Hewitt PE, Mackenzie J, Ironside JW, Galea G, et al. Investigation of variant Creutzfeldt-Jakob disease implicated organ or tissue transplantation in the United Kingdom. Transplantation. 2014;98:585–9. DOIPubMed
- Jayanthi P, Thomas P, Bindhu P, Krishnapillai R. Prion diseases in humans: oral and dental implications. N Am J Med Sci. 2013;5:399–403. DOIPubMed
- Peden A, McCardle L, Head MW, Love S, Ward HJ, Cousens SN, et al. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia. 2010;16:296–304. DOIPubMed
- Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–21. DOIPubMed
- Lefrère JJ, Hewitt P. From mad cows to sensible blood transfusion: the risk of prion transmission by labile blood components in the United Kingdom and in France. Transfusion. 2009;49:797–812. DOIPubMed
- Edgeworth JA, Farmer M, Sicilia A, Tavares P, Beck J, Campbell T, et al. Detection of prion infection in variant Creutzfeldt-Jakob disease: a blood-based assay. Lancet. 2011;377:487–93. DOIPubMed
- Jackson GS, Burk-Rafel J, Edgeworth JA, Sicilia A, Abdilahi S, Korteweg J, et al. A highly specific blood test for vCJD. Blood. 2014;123:452–3. DOIPubMed






















.png)











No hay comentarios:
Publicar un comentario