
Immune-Cell Traps May Aid Cancer Metastasis
December 6, 2016, by NCI Staff
Cancer cells are infamous for recruiting normal cells to help them grow and spread. Now a new study suggests that cancer cells may exploit a normal function of neutrophils, the most common form of white blood cell, to help form metastatic tumors.
Other studies have linked neutrophils to cancer metastasis and suggested how they might achieve this task. This new study, the authors believe, may help to fill in some of the blanks in this process.
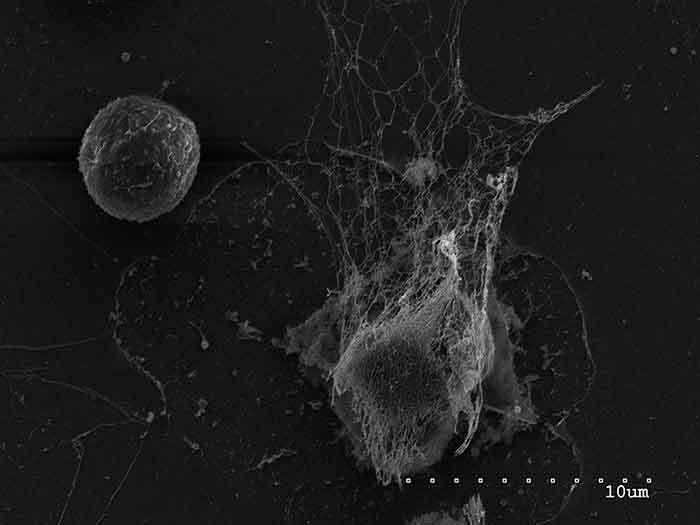
Cancer cells, the study suggests, can induce neutrophils to release special traps, which these immune cells typically use to capture and destroy pathogens. For cancer cells that escape from the original tumor and travel to a distant location in the body, these so-called neutrophil extracellular traps (NETs) appear to help the cancer cells form tumors, or metastases, in other tissues, the research team reported.
Led by investigators at Cold Spring Harbor Laboratory in New York, the researchers also developed a nanotechnology-based therapy that disintegrates NETs. The treatment, they showed, could prevent or greatly diminish the formation of metastatic tumors in the lungs of mice with an aggressive form of breast cancer.
Although the team has some theories about how NETs facilitate metastasis, exactly how this happens is still unclear, said the study’s lead author, Mikala Egeblad, Ph.D. But the findings suggest that “NETs are critical for metastatic colonization,” the researchers wrote in the October 19 Science Translational Medicine, and “raise the exciting possibility of targeting NETs to prevent metastasis.”
Casting NETs
As the first responders to potentially harmful bacteria and other pathogens, neutrophils are an important component of the human immune system. One of the ways that neutrophils rid the body of harmful pathogens is by forming NETs, a meshwork of DNA and toxic enzymes that trap and kill these invaders.
Over the last decade, the role of immune system cells in cancer—as both promoters of and impediments to tumor development, progression, and spread—have become of keen interest, explained Rosandra Kaplan, M.D., of NCI’s Center for Cancer Research, whose research focuses on the tumor microenvironment and its specific components, including immune cells, that promote metastasis.
But neutrophils have not received the same amount of attention as other immune cells, such as T cells and macrophages, she continued.
“T cells have really had the center stage. They’re the best at killing tumor cells, so they’ve received a lot of the focus,” Dr. Kaplan said. Neutrophils, on the other hand, don’t hang around very long in the body once they are produced and they aren’t as lethal as other immune cells, she continued, so their culpability in promoting cancer or prowess as an anti-tumor weapon has been less studied.
Dr. Egeblad and her team, following up on the earlier studies linking neutrophils to metastasis, focused initially on breast cancer metastasis to better understand how and where neutrophils might support this process.
For example, in mouse models of a highly metastatic breast cancer, they showed that neutrophils—and signaling molecules that recruit them—could be found in abundance in primary and metastatic tumors.
It wasn’t until they turned to an advanced imaging technology known as confocal intravital lung imaging (CILI), however, that the potential role of NETs became clear. Using CILI, the researchers scanned the lungs of mice that had been injected with breast cancer cells that preferentially spread to the lungs and saw structures jutting out from some cells. Additional experiments revealed that the structures were NETs.
And despite no evidence of infection in the lungs, the number of NETs in the mice remained “elevated for days” after the breast cancer cells were injected, they reported.
To see if NETs are present in human breast cancers, the researchers analyzed samples of primary and metastatic tumors from breast cancer patients. Although they found NETs in both types of tumor samples, the traps were found in the greatest abundance and most consistently in the tumor samples from women with the highly metastatic form of breast cancer known as triple negative.
Additional experiments in the lab showed that when neutrophils were grown in the presence of highly aggressive breast cancer cells, it led to robust NET formation and made the cancer cells behave far more aggressively. Neither of these outcomes occurred when they cultured neutrophils with breast cancer cells that don’t rapidly grow and spread.
Other experiments suggested that cancer cells might induce neutrophils to form and release NETs by turning on key signaling pathways in the immune cells.
Digesting NETs
The DNA-based structure of NETs, the researchers suspected, might present a vulnerability that could potentially be exploited—something that’s already done for people with cystic fibrosis (CF).
A hallmark of CF is a buildup in the lungs of a thick mucus caused in part by the accumulation of NETs triggered by persistent infections. So patients with CF are often treated with an inhaled drug that uses a DNA-digesting enzyme, called DNase I, to dissolve the traps and thin the mucus.
The enzyme showed strong hints of efficacy in cell lines of metastatic breast cancer. But when they tested DNase I in mouse breast cancer models, the enzyme’s effect on metastasis “was not very robust,” Dr. Egeblad said.
Based on data from older studies of DNase I, the research team speculated that the DNase I enzyme was rapidly degraded in the body and that this was the rate-limiting factor in the mice injected with metastatic breast cancer cells.
So they turned to Michael Goldberg, Ph.D., of the Dana-Farber Cancer Institute, whose lab had developed nanoparticles to which enzymes can be bound, allowing them to remain stable and active for longer periods.
The approach worked. In the first three mice treated with a nanoparticle to which the research team had bound DNase I, “we saw very striking effects,” Dr. Egeblad said.
Overall, 3 of the 9 mice treated with the nanoparticle-bound DNase I had no detectable metastases, and all of the mice treated with the drug had fewer metastases and markedly smaller metastases than mice treated with control nanoparticles lacking DNase I.
Understanding the Balance
There are still many unanswered questions about the roles of neutrophils, other immune cells, and other actors in the tumor microenvironment in both promoting and suppressing the growth or spread of tumors, Dr. Kaplan cautioned.
For example, although the finding that NETs might promote metastasis is “quite novel,” she continued, it’s consistent with findings from other studies that have implicated other compounds excreted by cells in the metastatic process, including long non-coding RNAs and small sacs called exosomes.
To complicate things further, Dr. Egeblad stressed, the available research strongly suggests that neutrophils or macrophages or other factors could aid tumor development in some situations and hinder it in others.
“It’s very likely that there is a fine balance” between when an immune cell fuels cancer development or inhibits it, she continued—a balance that is influenced by many different factors that researchers are just beginning to understand.
Her team is working to build on their findings, including conducting experiments that might help them better understand exactly how NETs facilitate the formation of metastatic tumors by cancer cells that have spread to a distant site. Even then, Dr. Egeblad cautioned, what they discover may have limitations.
“We know that tumors hijack different processes to promote metastasis,” she said. “We need to look at other cancer types and be open to the idea that they may not all be using the same process.”





















.png)












No hay comentarios:
Publicar un comentario