|
Nadia L. Oussayef, JD1; Satish K. Pillai, MD1; Margaret A. Honein, PhD1; C. Ben Beard, PhD1; Beth Bell, MD1; Coleen A. Boyle, PhD1; Lars M. Eisen, PhD1; Katrin Kohl, MD, PhD1; Matthew J. Kuehnert, MD1; Eva Lathrop, MD1; Stacey W. Martin, MSc1; Rebecca Martin, PhD1; Janet C. McAllister, PhD1; Elizabeth Pantino McClune, MSW, MPA1; Paul Mead, MD1; Dana Meaney-Delman, MD1; Brett Petersen, MD1; Lyle R. Petersen, MD1; Kara N.D. Polen, MPH1; Ann M. Powers, PhD1; Stephen C. Redd, MD1; James J. Sejvar, MD1; Tyler Sharp, PhD1; Julie Villanueva, PhD1; Denise J. Jamieson, MD1 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
The introduction of Zika virus into the Region of the Americas and the subsequent increase in cases of congenital microcephaly resulted in activation of CDC’s Emergency Operations Center and the declaration of a Public Health Emergency of International Concern by the World Health Organization. As of December 15, 2016, 61 countries and territories have reported local Zika virus transmission as part of the current outbreak; 29 countries and territories have reported potential cases of congenital Zika syndrome.
What is added by this report?
CDC’s emergency response to Zika virus rapidly addressed many acute public health needs associated with the outbreak and developed new public health surveillance and infection control tools, including issuing travel and clinical guidance; identifying sexual transmission; monitoring blood safety; developing and distributing laboratory test kits; establishing the causal link between in utero infection and congenital Zika syndrome and assessing the range of outcomes and the magnitude of risk; improving access to contraception to prevent unintended pregnancies; implementing vector control strategies; and improving the understanding of the link between and Zika virus infection and other neurological illnesses.
What are the implications for public health practice?
To protect pregnant women and their fetuses and infants from the effects of Zika virus infection during pregnancy, public health activities must focus on preventing mosquito-borne transmission through vector control and personal protective practices, preventing sexual transmission by advising abstention from sex or consistent and correct use of condoms, and preventing unintended pregnancies by reducing barriers to access to highly effective reversible contraception. Collectively, these critical strategies can reduce the effect of the virus on infants, families, and communities.
Nadia L. Oussayef, JD1; Satish K. Pillai, MD1; Margaret A. Honein, PhD1; C. Ben Beard, PhD1; Beth Bell, MD1; Coleen A. Boyle, PhD1; Lars M. Eisen, PhD1; Katrin Kohl, MD, PhD1; Matthew J. Kuehnert, MD1; Eva Lathrop, MD1; Stacey W. Martin, MSc1; Rebecca Martin, PhD1; Janet C. McAllister, PhD1; Elizabeth Pantino McClune, MSW, MPA1; Paul Mead, MD1; Dana Meaney-Delman, MD1; Brett Petersen, MD1; Lyle R. Petersen, MD1; Kara N.D. Polen, MPH1; Ann M. Powers, PhD1; Stephen C. Redd, MD1; James J. Sejvar, MD1; Tyler Sharp, PhD1; Julie Villanueva, PhD1; Denise J. Jamieson, MD1 (View author affiliations)
View suggested citation
The introduction of Zika virus into the Region of the Americas (Americas) and the subsequent increase in cases of congenital microcephaly resulted in activation of CDC’s Emergency Operations Center on January 22, 2016, to ensure a coordinated response and timely dissemination of information, and led the World Health Organization to declare a Public Health Emergency of International Concern on February 1, 2016. During the past year, public health agencies and researchers worldwide have collaborated to protect pregnant women, inform clinicians and the public, and advance knowledge about Zika virus (Figure 1). This report summarizes 10 important contributions toward addressing the threat posed by Zika virus in 2016. To protect pregnant women and their fetuses and infants from the effects of Zika virus infection during pregnancy, public health activities must focus on preventing mosquito-borne transmission through vector control and personal protective practices, preventing sexual transmission by advising abstention from sex or consistent and correct use of condoms, and preventing unintended pregnancies by reducing barriers to access to highly effective reversible contraception.
1. Issuing Travel Guidance to Warn Pregnant Women Not to Travel to Areas with Ongoing Zika Virus Transmission
On January 15, 2016, CDC issued a travel notice to alert travelers about the risk of Zika virus transmission in 14 countries and territories in Central and South America and the Caribbean, including Puerto Rico. As of December 15, 2016, a total of 60 international Zika travel notices have been issued, including 49 in the Americas. These notices advise pregnant women to avoid travel to areas of active Zika virus transmission, provide recommendations for travelers to avoid exposure to Zika virus, and inform returning travelers about transmission prevention and testing. On August 1, 2016, after the first instance of confirmed mosquito-borne Zika virus transmission in the continental United States, CDC issued domestic travel and diagnostic testing guidance for pregnant women and women of reproductive age living in or traveling to an area of Miami-Dade County, Florida (1). On November 28, 2016, local mosquito-borne transmission of Zika virus was reported in Brownsville (Cameron County), Texas, and on December 14, 2016, CDC issued guidance for travel and testing of pregnant women and women of reproductive age living in or traveling to Brownsville (2). CDC has continued to collaborate closely with state and local jurisdictions to determine when to issue, revise, or lift domestic travel guidance on the basis of epidemiologic evidence (3–5).
2. Publishing Clinical Guidance for the Care of Pregnant Women and Infants
As a newly recognized congenital infection, Zika virus presents unique challenges for obstetric and pediatric health care providers. CDC’s first Zika-related clinical guidance outlining evaluation, testing, and clinical management of Zika virus in pregnant women was released on January 19, 2016 (6), and on January 26, 2016, guidance for the evaluation and testing of infants with possible congenital Zika virus infection was released (7). As new evidence emerged, CDC updated pregnancy and infant guidance and developed guidance for reproductive-aged women (8–13). These evidence-based recommendations have been disseminated to health care providers through partnerships with professional organizations, including the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, and have provided clear guidance for providers monitoring and caring for pregnant women and fetuses and infants affected by Zika virus infection.
3. Identifying Sexual Transmission of Zika Virus Infection
In late January, CDC, in collaboration with Texas health officials, confirmed sexual contact as the source of Zika virus infection in a Dallas man whose partner had traveled to Brazil (14) and issued guidance for the prevention of sexual transmission of Zika virus in February (15). To date, 38 cases of sexually transmitted Zika virus infection have been confirmed in the United States (16). Most cases have involved transmission from symptomatic men to women (17); however, cases of male-to-male (14), female-to-male (18), and asymptomatic male-to-female (19) sexual transmission have also been documented. In April and May, CDC initiated two studies to determine the frequency and duration of Zika virus shedding in the semen and urine of infected men. CDC continues to work closely with state, local, and territorial health officials to identify and investigate possible cases of sexual transmission of Zika virus. As new information regarding sexual transmission emerges, one recommendation remains consistent: men who live in or have traveled to an area with active Zika virus transmission should prevent sexual transmission to their pregnant partners by abstaining from sex or consistently and correctly using condoms for the duration of their partner’s pregnancy (10,12,13,15).
4. Monitoring Blood Safety and Availability
Because of known transfusion-transmission risks associated with other flaviviruses, Zika virus was recognized as a potential threat to blood safety. The Food and Drug Administration (FDA) and CDC collaborated to recommend travel and risk factor–related deferrals for all U.S. blood donors; in February 2016, FDA issued guidance recommending that, until laboratory screening of blood donations or pathogen-reduction technology could be implemented, blood centers in areas with active mosquito-borne Zika virus transmission cease local blood collection and import blood from U.S. areas without active transmission (20). Because of ongoing local transmission of Zika virus in Puerto Rico and unavailability of either screening or pathogen-reduction technology for all blood products, CDC, in collaboration with the Puerto Rico Department of Health, conducted a rapid assessment of blood collection and use on the island to help guide blood importation measures (21). Blood importation, supported by the Biomedical Advanced Research and Development Authority, continued for Puerto Rico until April 2016, when Zika virus screening of blood donations was implemented under an FDA-approved investigational new drug application (21–23). Based on increasing reports of persons infected through travel as well as local transmission, FDA expanded its blood screening recommendations in August 2016 to include all areas of the United States (24). As of December 10, 2016, products from 78 donations in the continental United States and Hawaii and 353 donations in Puerto Rico have been prevented from entering the blood supply as a result of screening.
5. Developing and Distributing Laboratory Test Kits and Reagents
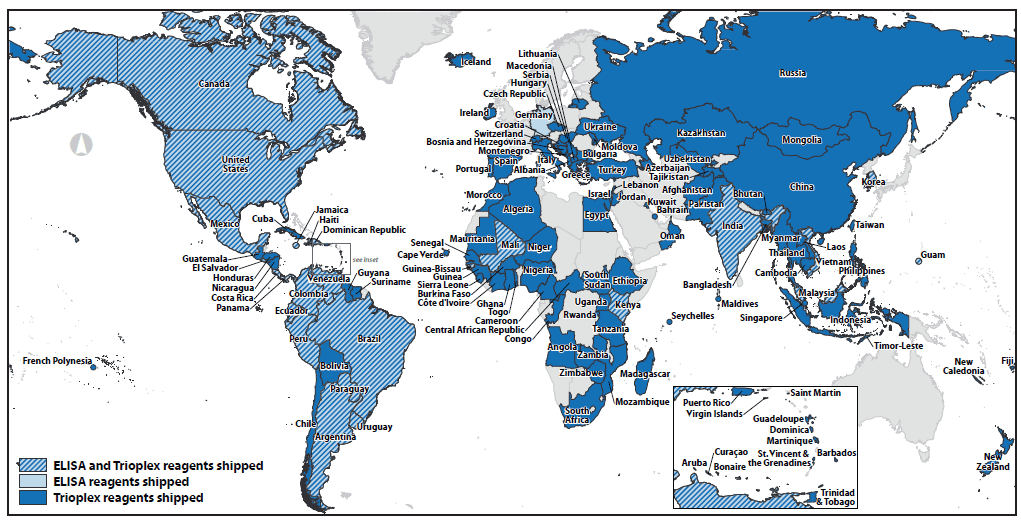
Working with FDA, CDC obtained the first two emergency use authorizations for CDC-developed in vitro tests to diagnose Zika virus infection: the Zika immunoglobulin M capture enzyme-linked immunosorbent assay (MAC-ELISA) on February 26, 2016 and the Trioplex real-time reverse transcription–polymerase chain reaction assay for the detection and differentiation of RNA from dengue, chikungunya, and Zika viruses on March 17, 2016. CDC manufactured and conducted quality control testing of reagents required for both tests and distributed them domestically and to approximately 100 countries (Figure 2). CDC continues to provide guidance to laboratories on all aspects of testing and interpretation of test results for all Zika virus emergency use authorization tests (25). CDC has continued to work to expand Zika immunoglobulin M testing capacity by entering into material transfer agreements and biologic material licensing agreements with commercial laboratories.
6. Establishing a Causal Link Between Zika Virus Infection During Pregnancy and Serious Brain Abnormalities, Including Microcephaly
In collaboration with colleagues from Brazil, CDC pathology experts identified the first evidence of Zika virus infection in the fetal brain and in placental tissues, providing evidence of the possible role of Zika virus infection during pregnancy in adverse fetal and infant outcomes (26,27). In April 2016, CDC authors published a comprehensive analysis of the data, concluding that sufficient evidence existed to support a causal relationship between Zika virus infection during pregnancy and microcephaly and other brain abnormalities (28). As of December 15, 2016, 29 countries and territories have reported potential cases of congenital Zika syndrome.*
7. Gathering and Analyzing Zika Pregnancy Surveillance Data to Understand the Magnitude of the Risk and the Full Range of Fetal and Infant Outcomes
To monitor the effect of Zika virus infection during pregnancy, pregnancy and infant surveillance was put in place in U.S. states and territories (29). The U.S. Zika Pregnancy Registry was established in coordination with state, local, tribal, and territorial health departments to monitor all states and territories except Puerto Rico. In Puerto Rico, the Zika Active Pregnancy Surveillance System was established to address specific needs resulting from the anticipated large outbreak (30). CDC also collaborated with the Instituto Nacional de Salud (National Institute of Health) in Colombia to conduct enhanced surveillance of pregnant women with symptomatic Zika virus disease in three cities (31). These surveillance systems continue to provide information about the magnitude of risk, the gestational timing of highest risk, and the spectrum of congenital Zika syndrome. Data reported to the U.S. Zika Pregnancy Registry from the continental United States and Hawaii suggest that among pregnant women with laboratory evidence of possible Zika virus infection, approximately 6% of fetuses or infants have a birth defect potentially related to Zika virus, and among women with first-trimester Zika infection, 11% of fetuses or infants have evidence of Zika-associated birth defects (32). The proportion of completed pregnancies affected by birth defects was similar following either symptomatic or asymptomatic infection during pregnancy (32). This estimate is consistent with models based on the outbreak in Bahia, Brazil, which estimated a 1%–13% risk for microcephaly after a Zika virus infection during the first trimester (33).
8. Improving Access to the Full Range of Voluntary, Reversible Contraception Methods to Decrease Unintended Pregnancies as a Strategy to Reduce the Impact of Zika Virus Infection
Prevention of unintended pregnancies is a primary strategy to reduce births of infants with Zika-related birth defects. A review of contraception use in Puerto Rico demonstrated limited supply, few trained providers, a cumbersome referral process, and limited provider reimbursement (34). The CDC Foundation, in collaboration with local partners and CDC, established the Zika Contraceptive Access Network (Z-CAN), with the aim of building a network of providers trained in contraception counseling and provision, securing sufficient contraceptive products to meet the needs of women in Puerto Rico, and raising awareness about the role of contraception in the context of Zika. By the end of 2016, among the 150 physicians actively providing obstetrical services in Puerto Rico, 105 (70%) had been trained and mentored on provision of all reversible methods of contraception. After approximately 3,000 initial Z-CAN visits, 96% of patients have received same-day contraceptive services, and 64% have chosen a long acting reversible contraceptive method. On August 2, 2016, CDC published a review of contraception use among women of reproductive age at risk for unintended pregnancy in states at potential risk for local Zika transmission; the review identified barriers to the use of highly effective contraception and described key strategies states can implement to increase access to contraception during periods of local Zika virus transmission (35).
9. Implementing Vector Control Strategies and Building the Evidence Base for Best Practices
Successful control of Aedes aegypti, the primary mosquito vector of Zika virus, has proven extremely difficult using existing control methods. CDC’s technical assistance during the Zika response has therefore included support for improved mosquito control infrastructure, novel mosquito control techniques, and integrated vector management that uses existing control methods. During the Zika virus outbreak in the Wynwood neighborhood of Miami-Dade County, Florida, aggressive ground-based integrated vector management was supplemented by sequential aerial applications of adulticide and larvicide, which rapidly reduced adult mosquito counts in surveillance traps by approximately 90% and helped to end this local outbreak (36). A similar approach in Miami Beach, Florida, using aerial applications of adulticide and ground-based applications of larvicide, also substantially reduced adult mosquito counts. Recent advances in aerial insecticide application methods, and the fact that, in the continental United States, Aedes aegypti lives primarily outdoors, likely contributed to the success of the aerial approach in Miami-Dade County. Public opposition to aerial pesticide application in Puerto Rico precluded a similar approach there; instead, lethal mosquito traps have been deployed as part of large community trials that aim to evaluate this method of preventing future outbreaks of mosquito-borne disease on the island (37).
10. Improving Understanding of the Link Between Guillain-Barré Syndrome and Zika Virus Infection
Many countries have reported increases in the occurrence of severe neurologic illness, particularly Guillain-Barré syndrome (GBS), after Zika virus outbreaks, with reported rates two to 10 times higher than those reported before Zika virus disease outbreaks (38–40). During the past year, the Puerto Rico Department of Health and CDC established an enhanced surveillance system for GBS in Puerto Rico. Initial analyses have demonstrated that among 56 patients with suspected GBS during January 1–July 31, 2016, a total of 26 (47%) had confirmed (n = 10, 18%) or presumptive (16, 29%) Zika virus infection (41). Other work related to GBS includes retrospective case-control investigations in Puerto Rico, Brazil, and Colombia, which will help improve understanding of the association between Zika virus infection and GBS.
Future Priorities
Zika virus remains a serious threat to world health that is likely to continue until a safe and effective vaccine becomes available and is widely implemented. Threats from mosquito-borne infection are likely to continue until better vector control interventions are developed. The severe consequences of Zika virus infection require a long-term approach with dedicated resources (42). Important future priorities include continuing to protect pregnant women and fetuses and infants from Zika virus infection; developing improved diagnostics, including the ability to distinguish among flaviviruses serologically; collaborating among government and private partners to accelerate vaccine development; developing and implementing improved vector surveillance and control strategies and capacities; improving contraceptive access to reduce unintended pregnancies; and improving understanding of the long-term outcomes for infants exposed to Zika virus in utero. Much remains to be done to protect pregnant women and fetuses and infants from Zika virus infection; the rapid action, dedication, and collaboration demonstrated by the global public health community during the past year provide a solid foundation for future work.
Corresponding author: Corresponding author: Satish K. Pillai, eocevent236@cdc.gov.
References
- CDC. CDC guidance for travel and testing of pregnant women and women of reproductive age for Zika virus infection related to the investigation for local mosquito-borne Zika virus transmission in Miami-Dade and Broward Counties, Florida. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://emergency.cdc.gov/han/han00393.asp
- CDC. CDC guidance for travel and testing of pregnant women and women of reproductive age for Zika virus infection related to the investigation for local mosquito-borne Zika virus transmission in Brownsville, Cameron County, Texas. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://emergency.cdc.gov/han/han00399.asp
- CDC. CDC expands guidance for travel and testing of pregnant women, women of reproductive age, and their partners for Zika virus infection related to mosquito-borne Zika virus transmission in Miami-Dade, Florida. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://emergency.cdc.gov/han/han00394.asp
- CDC. CDC updates guidance for travel and testing of pregnant women and women of reproductive age for Zika virus infection related to the ongoing investigation of local mosquito-borne Zika virus transmission in Miami-Dade County, Florida. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://emergency.cdc.gov/han/han00396.asp
- CDC. CDC updates guidance for pregnant women and women and men of reproductive age for Zika virus infection related to the ongoing investigation of local mosquito-borne Zika virus transmission in Miami-Dade County, Florida. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://emergency.cdc.gov/han/han00398.asp
- Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:30–3.CrossRef PubMed
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:63–7. CrossRef PubMed
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:122–7. CrossRef PubMed
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:315–22. CrossRef PubMed
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:323–5.CrossRef PubMed
- Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure—United States, July 2016. MMWR Morb Mortal Wkly Rep 2016;65:739–44. CrossRef PubMed
- Brooks JT, Friedman A, Kachur RE, LaFlam M, Peters PJ, Jamieson DJ. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, July 2016. MMWR Morb Mortal Wkly Rep 2016;65:745–7. CrossRef PubMed
- Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure—United States, September 2016. MMWR Morb Mortal Wkly Rep 2016;65:1077–81. CrossRef PubMed
- Deckard DT, Chung WM, Brooks JT, et al. Male-to-male sexual transmission of Zika virus—Texas, January 2016. MMWR Morb Mortal Wkly Rep 2016;65:372–4. CrossRef PubMed
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:120–1. CrossRefPubMed
- CDC. Zika virus: case counts in the US. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://www.cdc.gov/zika/geo/united-states.html
- Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:215–6. CrossRef PubMed
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus—New York City, 2016. MMWR Morb Mortal Wkly Rep 2016;65:716–7.CrossRef PubMed
- Brooks RB, Carlos MP, Myers RA, et al. Likely sexual transmission of Zika virus from a man with no symptoms of infection—Maryland, 2016. MMWR Morb Mortal Wkly Rep 2016;65:915–6.CrossRef PubMed
- Food and Drug Administration. Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2016. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf
- Vasquez AM, Sapiano MR, Basavaraju SV, Kuehnert MJ, Rivera-Garcia B. Survey of blood collection centers and implementation of guidance for prevention of transfusion-transmitted Zika virus infection—Puerto Rico, 2016. MMWR Morb Mortal Wkly Rep 2016;65:375–8. CrossRef PubMed
- Food and Drug Administration. FDA allows use of investigational test to screen blood donations for Zika virus. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm493081.htm
- Kuehnert MJ, Basavaraju SV, Moseley RR, et al. Screening of blood donations for Zika virus infection—Puerto Rico, April 3–June 11, 2016. MMWR Morb Mortal Wkly Rep 2016;65:627–8.CrossRef PubMed
- Food and Drug Administration. Revised recommendations for reducing the risk of Zika virus transmission by blood and blood components. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2016. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM518213.pdf
- CDC. Guidance for U.S. laboratories testing for Zika virus infection. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://www.cdc.gov/zika/pdfs/laboratory-guidance-zika.pdf
- Martines RB, Bhatnagar J, de Oliveira Ramos AM, et al. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet 2016;388:898–904. CrossRef PubMed
- Martines RB, Bhatnagar J, Keating MK, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016;65:159–60. CrossRef PubMed
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med 2016;374:1981–7. CrossRef PubMed
- Honein MA, Jamieson DJ. Monitoring and preventing congenital Zika syndrome. N Engl J Med 2016;375:2393–4. CrossRef PubMed
- Ellington SR, Devine O, Bertolli J, et al. Estimating the number of pregnant women infected with Zika virus and expected infants with microcephaly following the Zika virus outbreak in Puerto Rico, 2016. JAMA Pediatr 2016;170:940–5. CrossRef PubMed
- Cuevas EL, Tong VT, Rozo N, et al. Preliminary report of microcephaly potentially associated with Zika virus infection during pregnancy—Colombia, February–November 2016. MMWR Morb Mortal Wkly Rep 2016;65:1409–13. CrossRef PubMed
- Honein MA, Dawson A, Petersen EE, et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA Pediatr 2016. E-pub December 13, 20162016.
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med 2016;375:1–4. CrossRef PubMed
- Tepper NK, Goldberg HI, Bernal MI, et al. Estimating contraceptive needs and increasing access to contraception in response to the Zika virus disease outbreak—Puerto Rico, 2016. MMWR Morb Mortal Wkly Rep 2016;65:311–4. CrossRef PubMed
- Boulet SL, D’Angelo DV, Morrow B, et al. Contraceptive use among nonpregnant and postpartum women at risk for unintended pregnancy, and female high school students, in the context of Zika preparedness—United States, 2011–2013 and 2015. MMWR Morb Mortal Wkly Rep 2016;65:780–7. CrossRef PubMed
- Likos A, Griffin I, Bingham AM, et al. Local mosquito-borne transmission of Zika virus—Miami-Dade and Broward counties, Florida, June–August 2016. MMWR Morb Mortal Wkly Rep 2016;65:1032–8. CrossRef PubMed
- Lorenzi OD, Major C, Acevedo V, et al. Reduced Incidence of chikungunya virus infection in communities with ongoing Aedes Aegypti mosquito trap intervention studies—Salinas and Guayama, Puerto Rico, November 2015–February 2016. MMWR Morb Mortal Wkly Rep 2016;65:479–80. CrossRef PubMed
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016;387:1531–9.CrossRef PubMed
- dos Santos T, Rodriguez A, Almiron M, et al. Zika virus and the Guillain–Barré syndrome—case series from seven countries. N Engl J Med 2016;375:1598–601. CrossRef PubMed
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med 2016;374:1552–63. CrossRef PubMed
- Dirlikov E, Major CG, Mayshack M, et al. Guillain-Barré syndrome during ongoing Zika virus transmission—Puerto Rico, January 1–July 31, 2016. MMWR Morb Mortal Wkly Rep 2016;65:910–4. CrossRef PubMed
- World Health Organization. Statement on the 5th Meeting of the Emergency Committee under the International Health Regulations (2005) regarding microcephaly, other neurological disorders and Zika virus. Geneva, Switzerland: World Health Organization; 2016. http://www.who.int/mediacentre/news/statements/2016/zika-fifth-ec/en/
 FIGURE 1. Timeline of Zika virus response events, by month — worldwide, January–December 2016
FIGURE 1. Timeline of Zika virus response events, by month — worldwide, January–December 2016
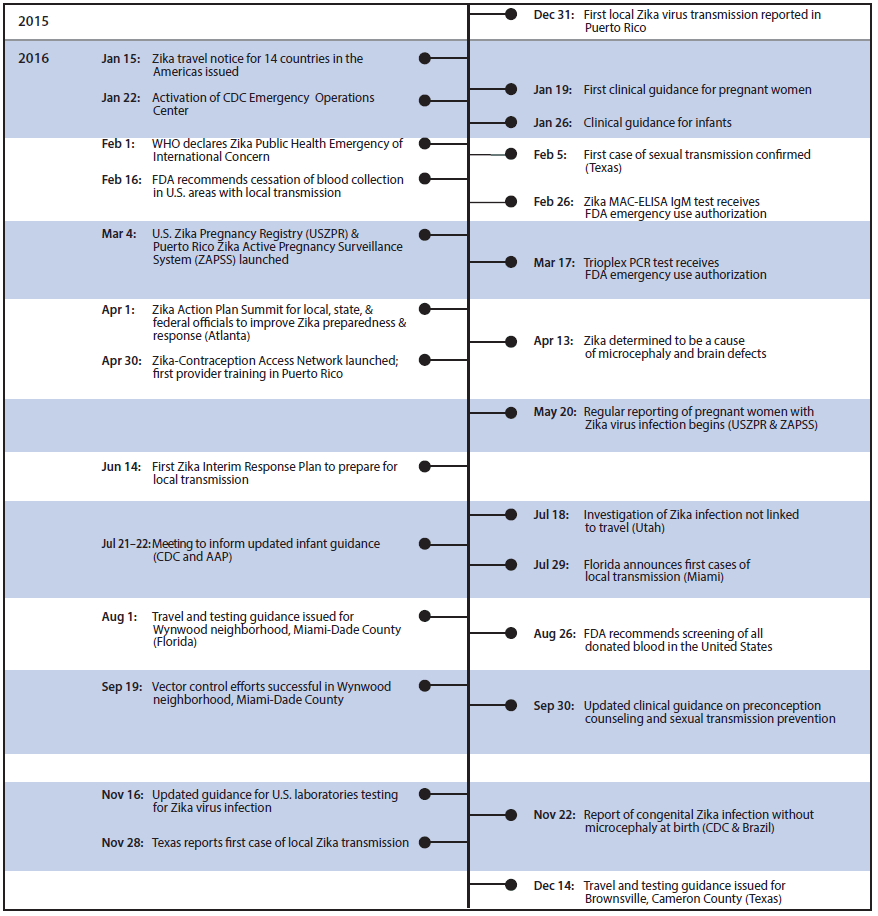
Abbreviations: AAP = American Academy of Pediatrics; FDA = Food and Drug Administration; MAC-ELISA = immunoglobulin M-capture enzyme-linked immunosorbent assay; PCR = polymerase chain reaction; WHO = World Health Organization.



































No hay comentarios:
Publicar un comentario