Volume 23, Number 6—June 2017
CME ACTIVITY - Research
Relative Risk for Ehrlichiosis and Lyme Disease in an Area Where Vectors for Both Are Sympatric, New Jersey, USA
On This Page
Abstract
The lone star tick, Amblyomma americanum, is a vector of Ehrlichia chaffeensis and E. ewingii, causal agents of human ehrlichiosis, and has demonstrated marked geographic expansion in recent years. A. americanum ticks often outnumber the vector of Lyme disease, Ixodes scapularis, where both ticks are sympatric, yet cases of Lyme disease far exceed ehrlichiosis cases. We quantified the risk for ehrlichiosis relative to Lyme disease by using relative tick encounter frequencies and infection rates for these 2 species in Monmouth County, New Jersey, USA. Our calculations predict >1 ehrlichiosis case for every 2 Lyme disease cases, >2 orders of magnitude higher than current case rates (e.g., 2 ehrlichiosis versus 439 Lyme disease cases in 2014). This result implies ehrlichiosis is grossly underreported (or misreported) or that many infections are asymptomatic. We recommend expansion of tickborne disease education in the Northeast United States to include human health risks posed by A. americanum ticks.
Tickborne diseases are a growing public health concern in the United States (1). Lyme disease, caused by the bacterium Borrelia burgdorferi, is the most frequently reported vectorborne illness in the Northeast (2) and has been the subject of widespread education campaigns aimed at preventing human encounters with its vector, Ixodes scapularis (the black-legged tick). Unfortunately, such campaigns focus comparatively less attention on other medically important ticks in Lyme disease–endemic areas (3); therefore, persons living in these areas may not fully recognize the threat posed by these species. Specifically, the much more aggressive lone star tick, Amblyomma americanum, transmits the agent of human monocytic ehrlichiosis and may serve as the vector for several other emerging tickborne pathogens (3–5). Historically found primarily in the Southeast United States but with established distributions along the Atlantic Coast and into the Midwest (6), A. americanum ticks are active concurrently with I. scapularis ticks; aggressively attack humans in all tick life stages (adult, nymph, and larvae); and are typically by far the more numerous of the 2 ticks where they are sympatric (7,8).

Figure 1. Number of ticks submitted each year to Monmouth County Mosquito Control Division’s passive surveillance program during May–August, by year, Monmouth County, New Jersey, USA, 2006–2015.
Given the abundance and aggressive host-seeking behavior of A. americanum ticks, it is reasonable to expect high rates of human encounters with them (8–10). In recent years, submissions of A. americanum ticks to the Monmouth County Mosquito Control Division’s passive tick surveillance program (Tinton Falls, New Jersey, USA; offering free tick identification to residents since 2006) have been increasing steadily while submissions of I. scapularis ticks remain nearly level (Figure 1), suggesting increased human exposure to A. americanum ticks relative to I. scapularis ticks.
A. americanum ticks are known vectors of >2 human pathogens, Ehrlichia chaffeensis and E. ewingii (10,11), which cause human ehrlichiosis, an illness often marked by an initial prodrome of undifferentiated fever, headache, myalgia, nausea, malaise, thrombocytopenia, leukopenia, and hepatic injury (elevated serum transaminase levels) (12). More severe complications (including toxic shock–like symptoms and meningitis or meningoencephalitis) may occur in some untreated persons (including immunocompromised patients), and death rates as high as ≈3% have been reported (12,13).
The number of ehrlichiosis cases attributable to E. chaffeensis that have been reported to the Centers for Disease Control and Prevention (Atlanta, GA, USA) has increased steadily since the disease became reportable, from 0.8 cases/million persons/year in 2000 to 3.0 cases/million persons/year in 2007 (13,14). However, some evidence suggests that ehrlichiosis may be substantially underreported (12) and even reported cases may be misclassified because of cross-reactivity between E. chaffeensis, E. ewingii, and other Ehrlichia and Anaplasma agents in widely used serologic tests (15–17). Health risks may be compounded if physicians are less familiar with ehrlichiosis than Lyme disease, particularly because initial symptoms may be relatively vague, resembling a viral syndrome typical of an array of tickborne diseases (14), which creates the potential for diagnostic confusion where I. scapularis and A. americanum ticks are sympatric (10). Ehrlichiosis could also be misdiagnosed as Rocky Mountain spotted fever if a patient is co-infected with Ehrlichia sp. and Rickettsia amblyommatis, (formerly Candidatus Rickettsia amblyommii [18]), a species cross-reactive in tests for rickettsial pathogens (19) and commonly present in >40% of field-collected A. americanum ticks (4).
This study quantified the risk to humans of ehrlichial infections, relative to Lyme disease risk. We used data obtained from tick surveillance programs in Monmouth County, New Jersey, an area with a high reported incidence of Lyme disease and increasing A. americanum tick encounter rates.
Site Description
Monmouth County (40°44′N, 74°17′W) is located in eastern-central New Jersey, a US state on the mid-Atlantic coast. The county is 468.8 mi2 in size and had a population of 630,380 (1,344.7 persons/mi2) as of the 2010 census (http://www.census.gov/quickfacts/table/PST045215/34025). The geomorphologic break separating the Inner and Outer Coastal Plain physiographic provinces in New Jersey runs horizontally across the center of the county, and the resulting soil differences are reflected in vegetative differences between these 2 regions (20). The Outer Coastal Plain region is characterized by sandier soils that are often dry and acidic, with pine forests and cedar swamps. Previously, the distribution of A. americanum ticks in Monmouth County was restricted to this southern part of the county (21), whereas I. scapularis ticks were found throughout. However, recently specimens of A. americanum ticks have been captured in the far north and west of the county.
The county reports several hundred cases of Lyme disease annually, with a 10-year average (during 2005–2014) of 361 cases/year. By contrast, during that same period, there were on average 5.5 cases/year of E. chaffeensis infection and no cases of infection attributed to E. ewingii (22).
Risk Model
The relative risk for infection with a tickborne pathogen depends on multiple factors, including risk for exposure to a competent vector, risk for exposure to the pathogen from exposure to the vector, and risk for transmission from exposure to a pathogen-infected vector. To characterize the risk for human exposure to ticks, we define the parameter Cx as the relative proportion of each species (x) of tick submitted to the Monmouth County Mosquito Control Division’s tick identification and testing service. This passive surveillance program, initiated in 2006, allows county residents to submit ticks they have encountered (e.g., found on their skin or clothing) for species identification. This program averages 658.9 submissions/year, although this number has increased markedly in recent years (R.A. Jordan, unpub. data). For Cx, we used the 10-year average of relative submissions data (2006–2015) during peak Lyme disease transmission season in New Jersey (May–August) (Table). Although in Monmouth A. americanum ticks are often 3 times as abundant as I. scapularis ticks in field collections (9), using the passive surveillance data in our model (where A. americanum ticks are only encountered 1.5 times as often; Table) provides a more direct measure of human exposure to ticks as well as a more conservative risk estimate.
To characterize risk for exposure to the disease from the vector, we define Ix as the prevalence of the pathogen in ticks (i.e., percentage infected), weighted by life stage. Both I. scapularis and A. americanum ticks have a 3-host life cycle, meaning that adults have had more opportunities to feed on an infected host than nymphs and consequently infection rates differ between life stages. Because transovarial transmission of either B. burgdorferi or E. chaffeensis does not occur (23,24), host-seeking larvae are not infected and therefore were not included in the calculations. Relative abundance of nymphs and adults of each species submitted to our passive tick surveillance program during May–August were reported (CX,N and CX,D) and used to weight the infection prevalence of each (Table). Infection rates of I. scapularis ticks with B. burgdorferi for both life stages (IIS,D and IIS,N) also were obtained from our passive surveillance program, whereby residents submitting an I. scapularis tick can elect to have it tested for B. burgdorferi through nested PCR assay (following established protocols [8]). Our records show that during a 10-year period of our program, 90.5% of residents submitting I. scapularis ticks during May–August have chosen to have them tested (R.A. Jordan, unpub. data), including 153 adults and 1,146 nymphs. However, the program does not test A. americanum ticks, so infection rates for this species were obtained from other sources. Rates of adult tick infection with E. chaffeensis and E. ewingii (IAA,D) in Monmouth County were derived from Schulze et al. (21) and summed, yielding a total value (accounting for co-infected ticks) of 11.7% infection with human Ehrlichia pathogens (N = 291). Nymphal infection rates with both ehrlichia species were obtained from an unpublished dataset consisting of field-collected nymphs from 4 sites in eastern and western Monmouth County in 2014 (R.A. Jordan unpub. data). Nymphal specimens were disrupted by using a TissueLyser and DNA isolated with QIAgen DNeasy 96 well-plate blood and tissue kits (QIAGEN, Valencia, CA, USA). Specimens were tested for pathogens by using real-time PCR protocols for E. chaffeensis (25) and E. ewingii (26). Both probes were modified slightly (shortened) to allow the use of an MGB quencher as follows (5′–3′): VIC-CGGACAATTGCTTATAACC-MGBNFQ for E. chaffeensis and 6FAM-AACAATTCCTAAATAGTCTCTGAC-MGBNFQ for E. ewingii. Sequence detection primers and reaction conditions were as described previously (26). A subset of samples was compared with conventional PCR methods established by this laboratory for detection of these pathogens (21), and the 2 methods were found to be in agreement. The resulting infection prevalence of Ehrlichia pathogens in A. americanum nymphs (IAA,N) (encompassing E. chaffeensis and E. ewingii, accounting for coinfection) was 9.04% (N = 752). Overall infection prevalence (Ix) in each species of tick, weighted by life stage, was calculated by multiplying the relative abundance of each life stage times its infection rate and summing across life stages (Table).
To characterize transmission risk, we defined TX as the likelihood of successful pathogen transmission from an infected tick to a human. The general concept of vector competence includes both this direction of transmission from vector to host as well as the probability of the vector becoming infected from feeding on infected hosts, which in our calculations is already reflected in tick infection rates (Ix). Data on transmission efficiency to animals for both species is scarce because most studies feed multiple infected ticks on 1 host (so the risk imposed by a single feeding tick is unknown) and, because of the difficulty in working with large vertebrates in a laboratory setting, have very small sample sizes (27–30). Further, whether probabilities of transmission obtained from animal studies are applicable to humans is unknown. Therefore, although we are including the parameter TX in the equation so that it can be applied when such data become better known, for purposes of these analyses we are setting it equal to 1 for both diseases, yielding no functional impact on the model.
Calculations
Based on our definitions, we calculate the relative risk for ehrlichiosis compared to Lyme disease as risk = (CAA × IAA × TAA)/(CIS × IIS × TIS). To then translate this value into expected cases of observed human infection, we move from risk to reported case numbers by using the reported number of cases of Lyme disease in Monmouth County as a benchmark. In other words, if there were X Lyme disease cases, then the expected number of ehrlichiosis cases would be X multiplied by the relative risk estimate calculated as described.
The relative risk for ehrlichiosis cases compared to Lyme disease was calculated as risk = (61.68 × 9.98 × 1)/(38.32 × 26.61 × 1) = 0.604. These numbers mean that we should expect to see ehrlichiosis cases occur 0.604 times as often as Lyme disease cases.
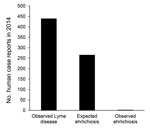
Figure 2. Number of observed versus expected ehrlichiosis cases, Monmouth County, New Jersey, USA, 2014. Expected values calculated by using number of observed Lyme disease cases as benchmark.
We demonstrate that in Monmouth County, New Jersey, ehrlichiosis infections from A. americanum ticks should be occurring, at a minimum, one half as often as Lyme disease (e.g., 1 ehrlichiosis case for every 2 Lyme disease cases). This rate of occurrence is clearly not the case (Figure 2), and these numbers suggest that ≈99% of potential ehrlichiosis infections are not recognized. It is possible that not all persons who become infected in Monmouth are county residents (and so their case would be recorded elsewhere) making the total number of Monmouth County–derived infections likely to be somewhat higher than the observed 2 cases. However, even if one assumes all ehrlichiosis reported for the entire state of New Jersey to originate in Monmouth County (64 cases in 2014) (22), these values still indicate a substantial discrepancy between numbers of observed and expected cases.
When selecting values for the parameters used in our calculations, every opportunity to be conservative was taken to avoid biasing estimates of relative risk. For example, infection (Ix) probabilities were higher for Lyme disease than ehrlichiosis (39.87% vs. 11.7% for adult ticks and 23.3% vs. 9.04% for nymphs). Although reported B. burgdorferi infection rates in I. scapularis adults frequently range from 40% to 50% in hyperendemic areas, several studies have reported lower rates (9,31). Our weighted infection prevalence estimate of 9.98% (encompassing both E. chaffeensis and E. ewingii across adults and nymphs) is probably lower than the actual value, given that many studies have reported infection prevalence in the range of 5%–15% for E. chaffeensis alone and in some locations twice that rate (3,32). Last, use of passive surveillance numbers (Cx) probably underestimates the actual risk for exposure to A. americanum ticks; residents who recognize the tick species may be less likely to bring in A. americanum ticks to the passive surveillance program, because we only test I. scapularis (as stated on our website), and taking the 10-year average for relative abundances (2006–2015) does not account for the recent surge in A. americanum tick submissions from 2012 onward (Figure 1). In light of these considerations, the actual risk for A. americanum tick–associated ehrlichioses in Monmouth County may be much higher than we have estimated.
One caveat should be noted. Differences in PCR sensitivity between B. burgdorferi and Ehrlichia sp. assays could affect our ability to compare infection rates, although to mitigate this problem as much as possible, we relied on established primers and checked that infection rates were within ranges reported by other studies as described previously. Thus, any difference in our ability to detect pathogens between the 2 tick species is unlikely to alter our conclusions.
One possible explanation for the lower-than-expected number of reported ehrlichiosis cases is a lack of awareness about ehrlichial disease on the part of the public and physicians, leading to misdiagnosis and underreporting. The infection tends to manifest as a general influenza-like illness, and onset of a rash is rare (12), so persons may be less likely to visit a doctor unless more severe symptoms emerge or they are aware of a recent tick bite and the presence of tickborne diseases in the area. Awareness of non–Lyme disease tickborne illnesses is startlingly low, even in parts of the country where ehrlichiosis cases outnumber Lyme disease cases (33–35). One study found that >50% of respondents in the United States had heard of Lyme disease, whereas only 1.4% had heard of ehrlichiosis (35). As a consequence of these factors, when active screening for ehrlichial infections is performed, the resulting case rates are often much higher than those reported to governmental agencies (3,36,37). For example, Olano et al. (37) found that the incidence of ehrlichiosis observed when actively screening patients was as much as 2 orders of magnitude higher than the passively reported incidence.
Another explanation could be the existence of asymptomatic infections. Several studies of E. chaffeensis antibody seroprevalence in adults found that most of those carrying the antibodies had no recollection of a symptomatic infection (38–40). Further, a study screening blood samples from children from the Southeast United States for E. chaffeensis revealed that many more children had been exposed than showed clinical signs (41). Most documented cases of E. chaffeensis and E. ewingii infection come from older adults (13,15), so when younger adults and children become infected, they may be less likely to have symptoms or seek treatment (41), accounting for the large number of unreported infections. Other vectorborne diseases, such as West Nile virus, transmitted by mosquitoes, also demonstrate large numbers of asymptomatic infections and increased severity among the older population (42). However, because of cross-reactivity in the serologic test for E. chaffeensis and the lack of specific testing for E. ewingii, one could argue that many presumed asymptomatic cases of E. chaffeensis were actually incidences of infection with E. ewingii, which tends to be more mild (16). Although our study considered E. chaffeensis and E. ewingii interchangeably, if we repeat our calculations by using E. chaffeensis infection rates alone, we would expect Monmouth County to have seen 84 cases of E. chaffeensis in 2014, when only 2 were reported. Therefore, 97.6% of E. chaffeensis infections are potentially going unnoticed (versus 99% of ehrlichiosis infections overall), which is still a troubling discrepancy.
Across the entire United States, the number of Lyme disease cases dwarfs ehrlichiosis cases. During 2004–2013, the Centers for Disease Control and Prevention annually reported 279–528 Lyme disease cases/1 million persons (2). In contrast, during that same period, annual ehrlichiosis cases ranged from 3.3 to 26 cases/1 million persons (43). If our numbers can be extrapolated to other areas in the country, this implies that, on a national scale, potentially thousands of ehrlichiosis cases are going undiagnosed. The A. americanum tick appears to be expanding into geographic areas where it did not occur previously (6,32) and is becoming more abundant within its existing range (9,21,44). Because ehrlichiosis cases have steadily increased since becoming reportable (13,15), the spread of A. americanum ticks and the emergence of ehrlichiosis as a human pathogen in the United States may parallel increases in I. scapularis tick populations and the emergence of Lyme disease that occurred 30 years prior (14). Even if most unrecognized infections are mild or asymptomatic, these could still have consequences for public health; for example, blood donors who are unknowingly infected could pass the infection to immunocompromised patients (45), or prescription of sulfa drugs for unrelated ailments could result in worsened disease presentation (46).
Our findings indicate a need to increase public education efforts about the risks for acquiring tickborne diseases other than Lyme disease in the United States, and in particular, to expand prevention awareness of medically important tick species other than I. scapularis. Fortunately, many of these diseases, including ehrlichiosis, can be prevented in the same manner as Lyme disease (e.g., avoidance of tick bites) and are treated similarly to Lyme disease (12,47); also, the number of ehrlichiosis cases peaks during spring and summer months, corresponding with peak Lyme disease transmission months (13,15). Consequently, refocusing existing public health education efforts to encompass the full spectrum of tickborne diseases could be accomplished without changing much of its content, although some attention should be given to the risks imposed by viruses and protozoa as well as unique characteristics of the questing behavior of A. americanum ticks. To better inform these educational efforts and more accurately assess tickborne disease risk, more research into diseases other than Lyme disease is required.
Humans can alter their environment in many ways that affect disease transmission, from localized changes affecting tick habitat and host abundance, to larger changes affecting the planet’s climate. As these changes continue to occur, the study of vector and pathogen distributions and abundance will be critical to understanding the potential risk to humans posed by emerging pathogens. Our calculations imply that infections with E. chaffeensis and E. ewingii are underrecognized, at least in Monmouth County, New Jersey, if not throughout a larger portion of the United States where A. americanum ticks are abundant or becoming abundant. Additional effort is needed to determine the causes for this apparent discrepancy and to characterize the actual prevalence of ehrlichiosis in the human population, as well as to raise awareness about the risk for exposure to these pathogens in areas where A. americanum ticks are common.
Dr. Egizi oversees a research laboratory for the Monmouth County Mosquito Control Division located in the Center for Vector Biology at Rutgers University in New Brunswick, New Jersey, USA. She is interested in using molecular tools to gain insight into the ecology and evolution of vectors and vectorborne disease in the northeastern United States.
Acknowledgment
The authors would like to acknowledge Terry L. Schulze and Holly Gaff for insightful discussions, Vivien E. Roegner for her role in laboratory analyses, and the comments of 2 anonymous reviewers that greatly improved this manuscript.
References
- Nadolny RM, Feldman KA, Pagac B, Stromdahl EY, Rutz H, Wee S-B, et al. Review of the Mid-Atlantic Tick Summit III: A model for regional information sharing. Ticks Tick Borne Dis. 2015;6:435–8. DOIPubMed
- Centers for Disease Control and Prevention. Lyme disease data and statistics 2005–2014. 2015 [cited 2015 Dec 4]. http://www.cdc.gov/lyme/stats/index.html
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–37. DOIPubMed
- Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, et al. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J Med Entomol. 2006;43:1261–8.PubMed
- Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, et al. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. DOIPubMed
- Springer YP, Eisen L, Beati L, James AM, Eisen RJ. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342–51. DOIPubMed
- Stromdahl EY, Randolph MP, O’Brien JJ, Gutierrez AG. Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) infection in Amblyomma americanum (Acari: Ixodidae) at Aberdeen Proving Ground, Maryland. J Med Entomol. 2000;37:349–56. DOIPubMed
- Schulze TL, Jordan RA, Schulze CJ, Mixson T, Papero M. Relative encounter frequencies and prevalence of selected Borrelia, Ehrlichia, and Anaplasma infections in Amblyomma americanum and Ixodes scapularis (Acari: Ixodidae) ticks from central New Jersey. J Med Entomol. 2005;42:450–6.PubMed
- Schulze TL, Jordan RA, Healy SP, Roegner VE, Meddis M, Jahn MB, et al. Relative abundance and prevalence of selected Borrelia infections in Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) from publicly owned lands in Monmouth County, New Jersey. J Med Entomol. 2006;43:1269–75. DOIPubMed
- Stromdahl EY, Hickling GJ. Beyond Lyme: aetiology of tick-borne human diseases with emphasis on the south-eastern United States. Zoonoses Public Health. 2012;59(Suppl 2):48–64. DOIPubMed
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet Parasitol. 2009;160:1–12. DOIPubMed
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(Suppl 1):S45–51. DOIPubMed
- Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg. 2011;85:124–31. DOIPubMed
- Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Rev. 2003;16:37–64. DOIPubMed
- Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing Incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94:52–60. DOIPubMed
- Harris RM, Couturier BA, Sample SC, Coulter KS, Casey KK, Schlaberg R. Expanded geographic distribution and clinical characteristics of Ehrlichia ewingii infections, United States. Emerg Infect Dis. 2016;22:862–5. DOIPubMed
- Dahlgren FS, Heitman KN, Behravesh CB. Undetermined human ehrlichiosis and anaplasmosis in the United States, 2008–2012: a catch-all for passive surveillance. Am J Trop Med Hyg. 2016;94:299–301. DOIPubMed
- Karpathy SE, Slater KS, Goldsmith CS, Nicholson WL, Paddock CD. Rickettsia amblyommatis sp. nov., a spotted fever group Rickettsia associated with multiple species of Amblyomma ticks in North, Central and South America. Int J Syst Evol Microbiol. 2016;66:5236–43. DOIPubMed
- Stromdahl EY, Jiang J, Vince M, Richards AL. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group Rickettsiae in the United States. Vector Borne Zoonotic Dis. 2011;11:969–77. DOIPubMed
- Collins BR, Anderson KH. Plant communities of New Jersey: a study in landscape diversity. New Brunswick (NJ): Rutgers University Press; 1994.
- Schulze TL, Jordan RA, White JC, Roegner VE, Healy SP. Geographical distribution and prevalence of selected Borrelia, Ehrlichia, and Rickettsia infections in Amblyomma americanum (Acari: Ixodidae) in New Jersey. J Am Mosq Control Assoc. 2011;27:236–44. DOIPubMed
- New Jersey Department of Health. 2005–2014 New Jersey Reportable Communicable Disease Reports [cited 2016 Mar 29]. https://www.nj.gov/health/cd/reportable_disease_stats.shtml
- Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis. 2013;4:46–51. DOIPubMed
- Long SW, Zhang X, Zhang J, Ruble RP, Teel P, Yu XJ. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2003;40:1000–4. DOIPubMed
- Loftis AD, Massung RF, Levin ML. Quantitative real-time PCR assay for detection of Ehrlichia chaffeensis. J Clin Microbiol. 2003;41:3870–2. DOIPubMed
- Killmaster LF, Loftis AD, Zemtsova GE, Levin ML. Detection of bacterial agents in Amblyomma americanum (Acari: Ixodidae) from Georgia, USA, and the use of a multiplex assay to differentiate Ehrlichia chaffeensis and Ehrlichia ewingii. J Med Entomol. 2014;51:868–72. DOIPubMed
- Unver A, Rikihisa Y, Stich RW, Ohashi N, Felek S. The omp-1 major outer membrane multigene family of Ehrlichia chaffeensis is differentially expressed in canine and tick hosts. Infect Immun. 2002;70:4701–4. DOIPubMed
- Jaworski DC, Bowen CJ, Wasala NB. A white-tailed deer/lone star tick model for studying transmission of Ehrlichia chaffeensis. Vector Borne Zoonotic Dis. 2013;13:193–5. DOIPubMed
- Starkey LA, Barrett AW, Chandrashekar R, Stillman BA, Tyrrell P, Thatcher B, et al. Development of antibodies to and PCR detection of Ehrlichia spp. in dogs following natural tick exposure. Vet Microbiol. 2014;173:379–84. DOIPubMed
- Varela-Stokes AS. Transmission of Ehrlichia chaffeensis from lone star ticks (Amblyomma americanum) to white-tailed deer (Odocoileus virginianus).J Wildl Dis. 2007;43:376–81. DOIPubMed
- Schulze TL, Jordan RA. The role of publicly owned properties in the transmission of Lyme disease in central New Jersey. Journal of Spirochetal and Tick-borne Diseases. 1996;3:124–9.
- Yabsley MJ. Natural history of Ehrlichia chaffeensis: vertebrate hosts and tick vectors from the United States and evidence for endemic transmission in other countries. Vet Parasitol. 2010;167:136–48. DOIPubMed
- Bayles BR, Evans G, Allan BF. Knowledge and prevention of tick-borne diseases vary across an urban-to-rural human land-use gradient. Ticks Tick Borne Dis. 2013;4:352–8. DOIPubMed
- Armstrong PM, Brunet LR, Spielman A, Telford SR III. Risk of Lyme disease: perceptions of residents of a Lone Star tick-infested community. Bull World Health Organ. 2001;79:916–25.PubMed
- Hook SA, Nelson CA, Mead PSUS. U.S. public’s experience with ticks and tick-borne diseases: Results from national HealthStyles surveys. Ticks Tick Borne Dis. 2015;6:483–8. DOIPubMed
- Walker DH. Ehrlichia under our noses and no one notices. In: Peters CJ, Calisher CH, editors. Infectious diseases from nature: mechanisms of viral emergence and persistence. Vienna: Springer-Verlag/Wien; 2004. p. 147–56.
- Olano JP, Masters E, Hogrefe W, Walker DH. Human monocytotropic ehrlichiosis, Missouri. Emerg Infect Dis. 2003;9:1579–86. DOIPubMed
- Yevich SJ, Sánchez JL, DeFraites RF, Rives CC, Dawson JE, Uhaa IJ, et al. Seroepidemiology of infections due to spotted fever group rickettsiae and Ehrlichia species in military personnel exposed in areas of the United States where such infections are endemic. J Infect Dis. 1995;171:1266–73. DOIPubMed
- Standaert SM, Dawson JE, Schaffner W, Childs JE, Biggie KL, Singleton J Jr, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420–5. DOIPubMed
- Fritz CL, Kjemtrup AM, Conrad PA, Flores GR, Campbell GL, Schriefer ME, et al. Seroepidemiology of emerging tickborne infectious diseases in a Northern California community. J Infect Dis. 1997;175:1432–9. DOIPubMed
- Marshall GS, Jacobs RF, Schutze GE, Paxton H, Buckingham SC, DeVincenzo JP, et al.; Tick-Borne Infections in Children Study Group. Ehrlichia chaffeensis seroprevalence among children in the southeast and south-central regions of the United States. Arch Pediatr Adolesc Med. 2002;156:166–70. DOIPubMed
- Huhn GD, Sejvar JJ, Montgomery SP, Dworkin MS. West Nile virus in the United States: an update on an emerging infectious disease. Am Fam Physician. 2003;68:653–60.PubMed
- Centers for Disease Control and Prevention. Ehrlichiosis: statistics and epidemiology, 1994–2010. 2015 [cited 2016 Jan 12]. http://www.cdc.gov/ehrlichiosis/stats
- Ginsberg HS, Ewing CP, O’Connell AF Jr, Bosler EM, Daley JG, Sayre MW. Increased population densities of Amblyomma americanum (Acari: Ixodidae) on Long Island, New York. J Parasitol. 1991;77:493–5. DOIPubMed
- Regan J, Matthias J, Green-Murphy A, Stanek D, Bertholf M, Pritt BS, et al. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis. 2013;56:e105–7. DOIPubMed
- Allen MB, Pritt BS, Sloan LM, Paddock CD, Musham CK, Ramos JM, et al. First reported case of Ehrlichia ewingii involving human bone marrow. J Clin Microbiol. 2014;52:4102–4. DOIPubMed
- Centers for Disease Control and Prevention. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Morb Mortal Wkly Rep. 2006;55(RR-4):1–29.PubMed






















.jpg)












No hay comentarios:
Publicar un comentario