
Measles vaccine protects against potentially serious illness

A Salvadoran nurse vaccinates a baby during a Task Force Northstar mission in El Salvador to provide medical care and other humanitarian and civic assistance. The mission involved U.S. military personnel working alongside their Brazilian, Canadian, Chilean, and Salvadoran counterparts. (U.S. Army Photo by Sgt. Kim Browne)
In the midst of a measles outbreak in the United States, public health officials are urging parents to get their children vaccinated, and for parents to make sure they're up to date on their own vaccinations.
As of March 28, there have been 387 confirmed cases of the potentially serious illness this year, according to the Centers for Disease Control and Prevention. In Rockland County, New York, officials announced a temporary ban on unvaccinated people gathering in enclosed public spaces, reporting that more than 80 percent of the individuals with measles had not been vaccinated.
Measles is a highly contagious virus that lives in the nose and throat mucus of an infected person. The virus, which is spread by air when an infected person breathes, coughs, or sneezes, can remain in the air for up to two hours after the infected individual leaves.
The vaccine to protect against measles is called the MMR vaccine because it also protects against mumps and rubella. Two doses of the vaccine are recommended, said Dr. Margaret Ryan, a retired Navy captain and the medical director of the Pacific Region Vaccine Safety Hub of the Defense Health Agency's Immunization Healthcare Branch.
According to the CDC, two doses of the MMR vaccine are about 97 percent effective in preventing measles, and one dose is about 93 percent effective.
"The MMR vaccine is a requirement for joining the service," Ryan said. "And all military family members should get the MMR vaccine along with other vaccines recommended by public health authorities such as the CDC. We strongly encourage and support protecting beneficiaries this way."
Noting that the DHA follows CDC recommendations, Ryan said children should get the first dose of the MMR vaccine at age 12 to 15 months. The second dose follows at least 28 days later, but usually between the ages of 4 and 6.
Adults who didn't receive two vaccine doses in childhood should also get at least one dose, she said.
"Those who are uncertain of their childhood vaccination history can get a blood test to confirm they're protected, or get the MMR vaccine," Ryan said. "Generally, it's safe to get extra doses."
The MMR vaccine is especially important for those who are traveling overseas. According to the World Health Organization, while deaths from measles have decreased significantly in recent years, the illness remains common, particularly in developing countries. According to the CDC, many of the recent U.S. cases of measles have been linked to visits to Israel, Ukraine, and other countries where large outbreaks have occurred.
People who should not get the vaccine include those who are pregnant, immune-compromised, or allergic to a component of the MMR vaccine, Ryan said. According to the CDC, allergic reactions are rare – about 1 in a million doses – and would occur anywhere from a few minutes to a few hours after the vaccination.
Some parents mistakenly believe the MMR vaccine is connected to autism spectrum disorders. However, a large body of scientific research has proven there is no link between vaccines and the developmental disorder, even in children at high risk.
While most people don't experience side effects from receiving the MMR vaccine, some may get a mild fever or redness, swelling, or pain at the injection site, Ryan said.
"These kinds of symptoms are less common after the second dose," she said, "and if they occur, they usually resolve over a few days."
Coming down with measles is uncomfortable at best. The illness causes a rash and high fever. Further, approximately 30 percent of measles patients have complications including pneumonia or encephalitis, which is inflammation of the brain. Ryan said about 1 in every 500 people who contract measles dies of the infection.
CDC data showed 372 cases in the civilian population in 2018. In 2017, there were no confirmed measles cases in the Military Health System, compared with 120 in the civilian population. The October 2017 Medical Surveillance Monthly Report includes data from 2010 to 2016 and can be accessed here.
Elmo comes to Madigan
Article
4/3/2019

Elmo began helping military kids and families with deployments and other military stressors in 2006
Pediatric clinic works to keep children healthy
Article
3/22/2019

The pediatric clinic’s objective is to care for children from birth to the age of 18
Medical helps prevent the flu amongst service members through teamwork
Article
2/15/2019

The Medical supply chain made approximately 3.4 million doses of the influenza vaccine available
Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger — United States, 2019
Report
2/8/2019
The 2019 child and adolescent immunization schedule summarizes the recommendations of the Advisory Committee on Immunization Practices (ACIP), including several changes from the 2018 immunization schedule.
Brush, clean in between to build a healthy smile
Article
2/5/2019
Children who have poor oral health often miss more school
III MEF Force Health Protection Requirements 2019
Policy
- Identification #: N/A
- Date: 2/1/2019
- Type: Directives
- Topics: Vaccine-Preventable Diseases | Vaccine Recommendations by AOR
Program Executive Office, Defense Healthcare Management Systems Fact Sheet
Fact Sheet
1/23/2019
This fact sheet provides an overview of the Program Executive Office, Defense Healthcare Management Systems (PEO DHMS) -- an acquisition organization that oversees three program management offices.
Report on Plan to Improve Pediatric Care and Related Services for Children of Members of the Armed Forces
Congressional Testimony
12/26/2018
HR 2810, NDAA Conference Report for FY 2018, Sec 733
Vaccination is the best defense against the flu
Article
12/10/2018
Vaccination is needed every year because the Influenza viruses change
Updated Framework for Development of Evidence-Based Recommendations by the Advisory Committee on Immunization Practices
Report
11/16/2018
In 2010, the Advisory Committee on Immunization Practices (ACIP) implemented the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for developing evidence-based recommendations. Since its February 2018 meeting, ACIP has incorporated the use of Evidence to Decision or Evidence to Recommendation (EtR) frameworks into GRADE methodology.
Global Routine Vaccination Coverage — 2017
Report
11/16/2018
Global coverage with vaccines to prevent diphtheria, tetanus, pertussis, polio, and measles has remained at 84%–85% since 2010. Prioritizing countries with the highest number of unvaccinated children to implement context-specific strategies has the potential to increase immunization coverage globally.
MILPERSMAN 1730-020 Immunization Exemptions for Religious Beliefs
Policy
Process for requesting immunization exemptions, pursuant to the Navy Military Personnel Manual.
- Identification #: MILPERSMAN 1730-020
- Date: 11/5/2018
- Type: Manual
- Topics: Vaccine-Preventable Diseases | Immunization Exemption Guidance
General Best Practice Guidelines for Immunization
Publication
10/30/2018
The Centers for Disease Control and Prevention (CDC) recommends routine vaccination to prevent 17 vaccine-preventable diseases that occur in infants, children, adolescents, or adults. This report provides information for clinicians and other health care providers about concerns that commonly arise when vaccinating persons of various ages.
Immunization Tool Kit 9th Edition
Publication
10/25/2018
A practical reference that facilitates and enhances the global delivery of quality immunization healthcare to Department of Defense (DoD) beneficiaries and employees. The Defense Health Agency Immunization Healthcare Branch (DHA-IHB) publishes the Immunization Tool Kit based on national recommendations, evidenced-based, peer-reviewed published medical ...
WRAIR begins Phase 1 clinical trial of Marburg vaccine
Article
10/19/2018
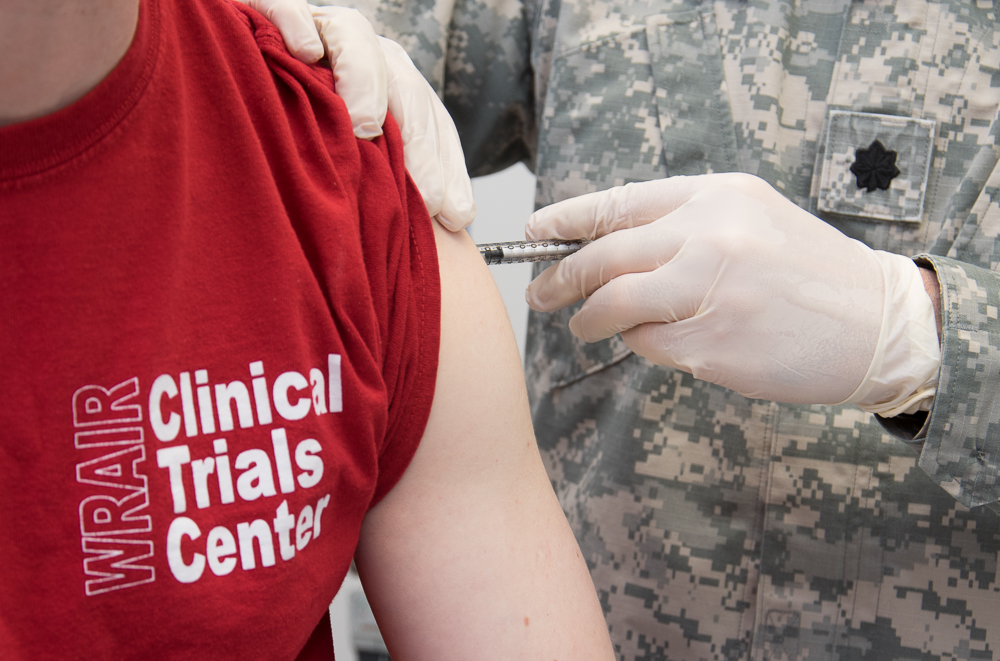
WRAIR recently administered the first vaccine in a Phase 1 clinical trial to evaluate the safety and immunogenicity of a Marburg vaccine





















.png)












No hay comentarios:
Publicar un comentario