Volume 24, Number 8—August 2018
Dispatch
Fatal Nongroupable Neisseria meningitidis Disease in Vaccinated Patient Receiving Eculizumab
On This Page
Deirdre Nolfi-Donegan , Monica Konar, Vianca Vianzon, Jessica MacNeil, James Cooper, Perrianne Lurie, Judi Sedivy, Xin Wang, Dan M. Granoff, and Lucy McNamara
, Monica Konar, Vianca Vianzon, Jessica MacNeil, James Cooper, Perrianne Lurie, Judi Sedivy, Xin Wang, Dan M. Granoff, and Lucy McNamara
Abstract
Patients receiving eculizumab have an increased risk for meningococcal disease, but most reported cases are attributable to encapsulated meningococcal strains. We describe a case in which a nongroupable meningococcal strain, which rarely causes disease in healthy persons, caused fatal disease in an eculizumab recipient despite meningococcal vaccination.
Eculizumab (Soliris; Alexion Pharmaceuticals, New Haven, CT, USA) is currently the only disease-modifying agent approved for patients with paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome. Eculizumab decreases complement-induced hemolysis by preventing cleavage of C5 into C5a and C5b; however, this activity also prevents formation of the membrane attack complex that is essential for meningococcal serum bactericidal activity (1).
The Food and Drug Administration–approved eculizumab prescribing information (2) includes a warning for increased risk for meningococcal disease and requires a Risk Evaluation and Mitigation Strategy (3) to ensure patient and prescriber awareness of the meningococcal disease risk and need for meningococcal vaccination. However, meningococcal disease occurs in eculizumab recipients despite appropriate vaccination (4–6). Most reported cases have been caused by encapsulated isolates. We describe a fully vaccinated patient with PNH who received 2 doses of eculizumab and died shortly thereafter from overwhelming Neisseria meningitidis disease caused by nongroupable meningococci, which rarely cause disease in human hosts.
In March 2016, a 16-year-old previously healthy girl was brought to a children’s hospital with abdominal pain, pancytopenia, and laboratory evidence of hemolysis. After extensive work-up, she received a diagnosis of PNH on the basis of peripheral blood flow cytometry. A bone marrow biopsy demonstrated cellularity ≈40%–50% and complete myeloid and erythroid maturation without evidence of dysplasia, aplasia, or an aberrant cell population. In anticipation of possible future eculizumab immunotherapy, she received a booster vaccine targeting N. meningitidis serogroups A, C, Y, and W-135 (Menactra; Sanofi Pasteur, Inc., Swiftwater, PA, USA) and a 2-dose series of vaccine targeting N. meningitidis serogroup B (MenB-4C) (Bexsero; GlaxoSmithKline, Bellaria Rosia, Sovicille, Italy).
During the first 4 months after diagnosis, the patient exhibited mild pancytopenia and compensated hemolysis but remained transfusion-independent. However, 6 months after PNH diagnosis, she began eculizumab treatment because of worsening symptoms.
Twenty-two hours after her second dose of eculizumab, the patient reported to a local emergency department with generalized body pain, headache, and emesis. She was afebrile, and her physical examination was unremarkable except for tachycardia of 124 beats per minute. Her leukocyte count was 8.9 × 109 cells/L (within reference limits); absolute neutrophil count was 8.46 × 109/L (upper limit 8.00 × 109 cells/L). Her symptoms were attributed to side effects from eculizumab and resolved after treatment with prochlorperazine, ketorolac, diphenhydramine, and intravenous fluids. She was discharged to home after 2 hours of observation.
Approximately 12 hours later, the patient reported weakness and purpura developed. She experienced cardiac arrest in transit to another emergency department, and resuscitative efforts were unsuccessful. Autopsy revealed hemorrhagic necrosis of the adrenal glands and focal hemorrhagic skin purpura, consistent with Waterhouse-Friderichsen syndrome.
By whole-genome sequencing, the meningococcal strain isolated from the meninges was found to be sequence type (ST) 2578 (clonal complex ST-41/44) and nongroupable with a capsule null locus, cnl. The inferred amino acid sequences of Factor H binding protein (FHbp) (peptide ID 100) and Neisseria heparin binding antigen (NHba) (peptide ID 2) were 97% and 100% identical to the respective antigens in the MenB-4C vaccine the patient had received, and both antigens were expressed on the surface of live bacteria based on flow cytometry. The remaining MenB-4C antigens were either absent (NadA) or mismatched (strain PorA P1,17,9).
Using 15% IgG-depleted human serum as a complement source, we found the strain was susceptible (titer >40) to bactericidal activity of mouse antiserum to recombinant FHbp ID 1, the subfamily B subvariant in the MenB-4C vaccine but not to mouse antiserum to NadA (not expressed by the strain) or to FHbp ID 22 (a subfamily A subvariant not in the vaccine) (titers <10). Mouse anti-NHba antiserum had a titer of <10. The relative resistance to anti-NHba was similar to previous findings that some NHba-expressing serogroup B meningococcal strains resist MenB-4C vaccine–elicited anti-NHba serum bactericidal activity (SBA) in humans (7).
A postmortem serum sample had high IgG reactivity against FHbp and NHba, both expressed by the infecting strain, and low reactivity to NadA (Figure 1). The high IgG reactivity specific for the 2 vaccine antigens expressed by the strain, but not for the vaccine antigen absent in the strain, suggests these antibodies may represent an IgG memory response elicited by infection. Given the short duration of symptoms, the likely stimulus for the memory antibody response would be asymptomatic nasopharyngeal colonization before disease onset.
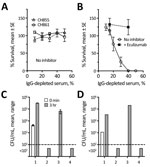
Figure 2. Effect of eculizumab on serum bactericidal activity and killing of Neisseria meningitidis by anticoagulated human blood. A) Complement-mediated bactericidal activity of an IgG-depleted human serum pool from 3 unvaccinated adult donors...
We also measured bactericidal activity against the patient isolate using pooled IgG-depleted serum from 3 healthy unvaccinated adults, performed as previously described (9). Whereas 2 invasive serogroup B encapsulated strains survived in the IgG-depleted serum pool (Figure 2, panel A), the nongroupable case-strain was killed (Figure 2, panel B), which could be from complement alone or complement activated by naturally acquired IgM. However, 50 µg/mL of eculizumab (a concentration less than or equal to trough serum levels in treated patients [10]) completely blocked killing of the case-isolate (Figure 2, panel B).
Finally, we tested eculizumab’s effect on whole blood killing of the unencapsulated case-isolate in an assay that measures a combination of SBA and opsonophagoctyic (OPA) activity (11,12). Cultures were sterile after 1–3 hours incubation in anticoagulated whole blood from MenB-4C–vaccinated or –unvaccinated adults (Figure 2, panels C, D), but 50 µg/mL eculizumab completely blocked bacterial killing. In contrast, the addition of a mouse monoclonal antibody to C7 (required for SBA but not OPA) did not prevent killing. These findings extend prior studies of whole blood killing of encapsulated serogroup B and C strains, which demonstrated that blocking C5 cleavage inhibited both SBA and OPA (11,12) killing of these strains, to a nonencapsulated meningococcal strain.
Collectively, these data indicate that eculizumab-treated patients remain profoundly susceptible to meningococcal disease, including from nongroupable meningococcal strains. Neither prior vaccination with MenB-4C, which matched 2 antigens present in the strain, nor the high serum antibody levels to FHbp and NHba in the patient prevented rapidly fatal disease in the presence of eculizumab. Similarly, eculizumab blocked killing of the nongroupable isolate by whole blood from healthy unvaccinated and vaccinated adults.
This case, along with several additional cases of meningococcal disease caused by nongroupable strains, was recently reported in eculizumab recipients in the United States (13). In otherwise healthy hosts, virtually all invasive meningococcal disease is caused by encapsulated strains (14). In contrast, unencapsulated strains are commonly associated with asymptomatic nasopharyngeal carriage. The sequence type of the isolate from this case, ST-2578, is rarely found in the PubMLST Neisseria database but previously has been observed primarily among isolates from asymptomatic carriers (15). Furthermore, the strain was killed by complement in human serum that had been depleted of IgG and by whole blood from an unvaccinated adult, features of commensal meningococcal strains that lack capsules.
In the United States, there is no official guidance on the use of antimicrobial chemoprophylaxis in eculizumab recipients. However, France and the United Kingdom recommend chemoprophylaxis for the duration of eculizumab therapy (references 16,17 in Technical Appendix[PDF - 266 KB - 1 page]), consistent with recommendations in recent case reports (1,13; reference 18 in Technical Appendix[PDF - 266 KB - 1 page]). Most chemoprophylactic regimens for eculizumab-treated patients use penicillin or erythromycin (1; reference 18 in Technical Appendix[PDF - 266 KB - 1 page]). Although the strain isolated from the patient reported was susceptible to penicillin, meningococcal disease has been reported in eculizumab patients receiving penicillin chemoprophylaxis caused by strains with penicillin resistance or intermediate sensitivity (5; reference 19 in Technical Appendix[PDF - 266 KB - 1 page]). These breakthrough cases underscore the need for healthcare providers and patients to have a high index of suspicion for meningococcal disease, leading to quick recognition and consideration of early empiric treatment, regardless of the patient’s vaccination status or chemoprophylactic regimen.
Dr. Nolfi-Donegan is a pediatric hematology-oncology fellow at Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center in Pittsburgh, Pennsylvania. Her primary research interests are coagulopathies and platelet disorders.
Acknowledgments
We thank Jarad Schiffer, Gowrisankar Rajam, and Cheryl Ellie for assistance with relevant assays not described here; the Centers for Disease Control and Prevention for characterizing the strain; and Jennifer Picarsic for her contribution to the pathology findings on autopsy.
This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (R01 AI046464 and R01 AI114701 to D.M.G.). The laboratory work was performed in a facility funded by the Research Facilities Improvement Program grant from the National Center for Research Resources, National Institutes of Health (grant C06 RR016226).
References
- Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, et al.; HUS International. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39. DOIPubMed
- Alexion Pharmaceuticals, Inc. Soliris. Revised 1/2016 [package insert] [cited 2016 Dec 2]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125166s417lbl.pdf
- Alexion Pharmaceuticals, Inc. Soliris REMS (Risk Evaluation and Mitigation Strategy) [cited 2017 Jul 23]. http://www.solirisrems.com
- Lebel E, Trahtemberg U, Block C, Zelig O, Elinav H. Post-eculizumab meningococcaemia in vaccinated patients. Clin Microbiol Infect. 2018;24:89–90. DOIPubMed
- Cullinan N, Gorman KM, Riordan M, Waldron M, Goodship TH, Awan A. Case report: Benefits and challenges of long-term eculizumab in atypical hemolytic uremic syndrome. Pediatrics. 2015;135:e1506–9. DOIPubMed
- Struijk GH, Bouts AH, Rijkers GT, Kuin EA, ten Berge IJ, Bemelman FJ. Meningococcal sepsis complicating eculizumab treatment despite prior vaccination. Am J Transplant. 2013;13:819–20. DOIPubMed
- Partridge E, Lujan E, Giuntini S, Vu DM, Granoff DM. The role of anti-NHba antibody in bactericidal activity elicited by the meningococcal serogroup B vaccine, MenB-4C. Vaccine. 2017;35:4236–44. DOIPubMed
- Lujan E, Winter K, Rovaris J, Liu Q, Granoff DM. Serum bactericidal antibody responses of students immunized with a meningococcal serogroup B vaccine in response to an outbreak on a university campus. Clin Infect Dis. 2017;65:1112–9. DOIPubMed
- Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, et al. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol. 2011;186:3606–14. DOIPubMed
- Hillmen P, Hall C, Marsh JC, Elebute M, Bombara MP, Petro BE, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria.N Engl J Med. 2004;350:552–9. DOIPubMed
- Konar M, Granoff DM. Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults. Blood. 2017;130:891–9. DOIPubMed
- Sprong T, Brandtzaeg P, Fung M, Pharo AM, Høiby EA, Michaelsen TE, et al. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood. 2003;102:3702–10. DOIPubMed
- McNamara LATN, Topaz N, Wang X, Hariri S, Fox L, MacNeil JR. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734–7. DOIPubMed
- Yazdankhah SP, Kriz P, Tzanakaki G, Kremastinou J, Kalmusova J, Musilek M, et al. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J Clin Microbiol. 2004;42:5146–53. DOIPubMed
- Marsh JW, Shutt KA, Pajon R, Tulenko MM, Liu S, Hollick RA, et al. Diversity of factor H-binding protein in Neisseria meningitidis carriage isolates. Vaccine. 2011;29:6049–58. DOIPubMed



































No hay comentarios:
Publicar un comentario