
Study Tracks the Evolution of Treatment Resistance in Metastatic Breast Cancer
November 7, 2017, by NCI Staff
Findings from a new study provide fresh insights into the treatment of women with metastatic breast cancer whose tumors are no longer responding to available treatments.
The cells in these treatment-resistant tumors, the study suggests, share some important characteristics that could potentially make the tumors vulnerable to therapies that otherwise might not have been considered.
In the study—which was funded by NCI’s Cancer Systems Biology Consortium—researchers performed comprehensive analyses of cancer cells from women with metastatic breast cancer that were collected serially throughout the course of their cancer treatment. The analyses, which included those on single cells, allowed the investigators to identify differences in individual cancer cells from the same patient, including pinpointing cells that were resistant or sensitive to treatment.
In general, their analyses showed that treatment-resistant cells shared some common, important functional characteristics—known as their phenotype—explained the study’s senior investigator, Andrea Bild, Ph.D., of the City of Hope Comprehensive Cancer Center in California.
In laboratory studies, the research team showed that, guided by the phenotype of the treatment-resistant cells, they could successfully kill those cells with targeted therapies.
Dr. Bild and her colleagues reported their findings November 1 in Nature Communications.
Much more research is needed before this approach for identifying treatment-resistant cell populations could be applied to patient treatment, Dr. Bild cautioned.
But, she said, “hopefully, with this information, we can eventually use therapies that target specific [tumor] phenotypes, in addition to those that work on specific genetic mutations, and treat cancers in a more targeted manner.”
Understanding Tumor Heterogeneity
Even when a cancer treatment initially works in a patient—by shrinking tumors or keeping them from growing further—the tumor cells can become resistant to it over time. Researchers have found that this resistance occurs when cancer cells evolve to develop ways to circumvent the mechanisms by which the treatment works.
An important contributor to treatment resistance is the phenomenon known as tumor heterogeneity. The cells in tumors are not identical to one another. Instead, studies have shown that different cells can possess different genetic alterations and rely on different signaling pathways to survive, grow, and spread. As a result, they may respond differently to different drugs.
There is a growing appreciation among researchers for the role that tumor heterogeneity plays in treatment resistance, explained Shannon Hughes, Ph.D., of NCI’s Division of Cancer Biology.
However, Dr. Hughes continued, the extent to which tumor cell heterogeneity influences treatment resistance “may vary from tumor type to tumor type, and also by the treatments used.”
And during the life of the cells in a tumor, that heterogeneity may also evolve, Dr. Bild stressed.
“What we don’t know—and what’s a really important question—is: As tumors are progressing, how is that [tumor cell] heterogeneity progressing?” she said.
Evolution of Tumor Subclones During Cancer Treatment
For the study, the researchers analyzed samples from four patients with metastatic breast cancer for whom samples were available from before their first treatment and over the course of subsequent treatments—which spanned several years in three women and nearly 15 in the other.
To be able to collect serial samples of tumors, the researchers relied largely on samples of fluid that often collects in the lungs or peritoneal cavity of patients with metastatic breast cancer.
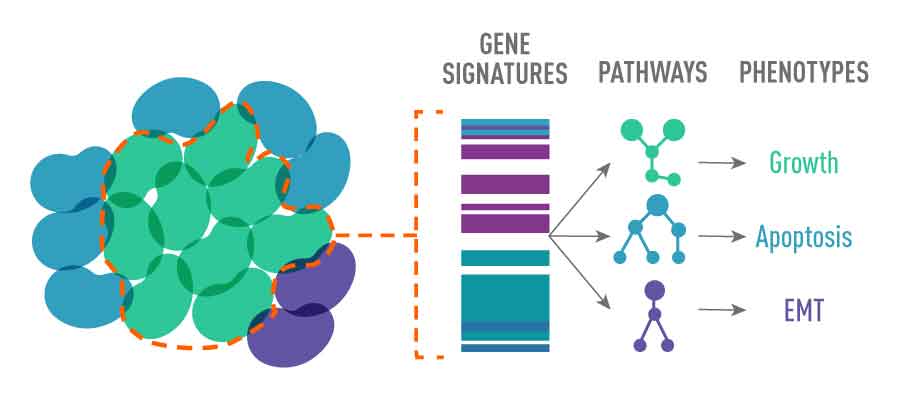
First, they performed genomic DNA sequencing to identify individual cells within a patient’s cancer that contain different genetic mutations and that are different from each other in other ways, called subclonal populations. They then analyzed individual cancer cells from the samples using a technology called single-cell RNA sequencing, which provides information on the phenotype of each subclone population.
This analysis allowed the researchers to identify the unique phenotype of different tumor subclones, namely what signaling pathways they were using—such as those that control the cells' ability to grow, blunt the immune response, or influence their ability to spread.
In so doing, they could trace the evolution of the phenotypes of treatment-resistant subclones throughout the course of each patient’s treatment.
The cancer cells’ evolution followed a similar pattern in all four patients: As the cells that were sensitive to a given cancer treatment died off, surviving treatment-resistant subclones became the predominant tumor cell populations. When a new treatment was tried, it often worked for a time, too, until other populations of resistant subclones formed and came to dominate the tumor.
In three of four cases, as the patients transitioned from treatment to treatment, the research, led by Sam Brady, Ph.D., identified what they called “bottleneck events,” in which just a single resistant population of cells predominated, often near the end of the patient’s life.
The results helped to answer an important question, Dr. Bild said.
“As the cancer became resistant to treatment, we were unsure if we would find an increased number of new tumor subclones or if there would be a limited number of resistant subclones,” she explained.
Guiding Cancer Treatment Based on Signaling Pathways
Different treatment-resistant subclones, the researchers found, had a particular reliance on common signaling pathways, including those involved in cell growth and differentiation, and those that can dial down the body’s immune response.
A Systems Approach to Studying Cancer Biology
NCI launched the Cancer Systems Biology Consortium (CSBC) in 2016, awarding grants to investigators to address important issues in cancer from a specific perspective: analyzing cancer as a complex biological system—essentially as an organ.
The systems approach relies on many of the same tools used to identify genetic alterations and other factors that affect the development and progression of tumors. But it also uses computational, mathematical, and other approaches to study cancer as an intricate network that is influenced by multiple factors, including genes, signaling pathways, and interactions with other cells and other factors in and around the tumor, Dr. Hughes explained.
The nine research centers that are currently funded through the CSBC are focused on addressing some of the biggest challenges facing clinicians and patients, she continued, including metastasis, treatment resistance, and interactions between the immune system and tumors.
“The teams use very quantitative approaches in their work,” Dr. Hughes said. For example, CSBC-funded researchers often develop mathematical models “that allow them to integrate many different types of data to make a prediction regarding an important biological process.”
For example, the treatment-resistant cells that remained after a bottleneck event often had increased activity of pathways that control a family of enzymes called receptor tyrosine kinases. Several receptor tyrosine kinases have been found to drive cancer progression. Therapies that target some of these enzymes are approved by the Food and Drug Administration, and others are being tested in clinical trials.
In lab experiments, treatment-resistant subclones from a patient who had developed resistance to the chemotherapy drug doxorubicin were highly sensitive to a combination of two different tyrosine kinase inhibitors, whereas cells collected from the patient before doxorubicin treatment were not affected at all by the drugs.
The findings, Dr. Bild and her colleagues wrote, suggest that the development of resistance “to one treatment may be associated with enhanced sensitivity to an alternative treatment targeting post-treatment phenotypes.”
Dr. Hughes noted that the findings from the new study are preliminary. But she stressed that they are important because they demonstrate that the broader approach used in the study, which analyzes tumors as a biological system—rather than as a homogenous mass of cells—can provide new insights into the biology of disease and potentially new treatment options.
The researchers “have taken a [signaling] pathway-centric view, compared with a gene-specific view,” Dr. Hughes said. “So if there is a pathway that’s commonly active across many cancers… if we can figure out how to target that pathway, we might be able to have an effect on many cancer types and many patients.”
The next step for this research is already underway, Dr. Bild said.
With CSBC funding, she and her colleagues will be analyzing how certain drug combinations work in patients whose tumors have specific subclone phenotypes. Their studies are embedded into clinical trials that are testing these drug combinations.
“We’re linking this approach to novel clinical trials to try to move the work forward to more clinically applicable approaches,” Dr. Bild said.






















.png)











No hay comentarios:
Publicar un comentario