Volume 25, Number 1—January 2019
Dispatch
Dengue Virus IgM Serotyping by ELISA with Recombinant Mutant Envelope Proteins
On This Page
Alexandra Rockstroh, Luisa Barzon, Widuranga Kumbukgolla, Hoang Xuan Su, Erley Lizarazo, Maria Fernanda Vincenti-Gonzalez, Adriana Tami, Alice M.M. Ornelas, Renato Santana Aguiar, Daniel Cadar, Jonas Schmidt-Chanasit, and Sebastian Ulbert
Abstract
We developed an IgM-based ELISA that identifies the dengue virus serotype of recent infections. Dominant serotypes were detectable in 91.1% of samples from travelers and 86.5% of samples from residents of endemic regions; 97.1% corresponded to the serotype identified by PCR. This ELISA enables more accurate reporting of epidemiologic findings.
Dengue virus (DENV) is an arthropodborne flavivirus that is endemic in tropical and subtropical regions, causing hundreds of millions of infections annually (1). It is subdivided into 4 serotypes, DENV-1–4. After infection, patients have lifelong immunity against the homologous serotype but remain susceptible to infections with the others (2). Such secondary infections have been shown to be a risk factor for severe dengue with life-threatening clinical manifestations, including dengue hemorrhagic fever or dengue shock syndrome (3). Thus, monitoring the serotype is essential for outbreak management, epidemiologic studies, and patient care. Analyses are often performed by using direct virus detection methods, such as PCR and nonstructural protein 1 (NS1) antigen capture (4). Despite the high specificities of these assays, their main disadvantages include a rather small diagnostic window for detection and, for NS1 antigen capture tests, low sensitivities during secondary DENV infections (5). Identification of the infecting serotype by serologic methods is hampered by the cross-reactivity of antibodies elicited by the immune response against flaviviruses (6). Previous reports have shown that the insertion of mutations in the conserved fusion loop domain of flavivirus envelope proteins reduces this cross-reactivity in diagnostic testing (7–9). Using this method, we developed a DENV-specific ELISA capable of differentiating DENV from other clinically relevant flaviviruses, such as Zika virus, West Nile virus, and tick-borne encephalitis virus (10). In this study, we evaluated the potential of this technique to distinguish the 4 DENV serotypes during the acute phase of infection on the basis of IgM detection.
We acquired DENV PCR–confirmed and, thereby, serotype-classified serum samples that we divided into 2 groups: those from returning travelers with residence in Italy or Germany (n = 45), collected from patients 5–60 days after symptom onset during 2013–2016; and those from persons residing in the DENV-endemic countries of Sri Lanka (n = 43), Vietnam (n = 24), Venezuela (n = 5), and Brazil (n = 2), collected from patients 2–8 days after symptom onset during 2013–2018. We also had a set of 14 DENV PCR–negative but NS1-positive (PLATEILA DENGUE NS1 AG; Bio-Rad, Hercules, CA, USA) serum samples from patients in Vietnam that were collected during the same outbreak as the other patients from Vietnam with DENV PCR–positive test results. Ethics approvals were obtained from the respective local authorities for all samples.
We pretested all serum samples with a DENV-specific ELISA that used 4 recombinant DENV envelope proteins (1 per serotype) containing 4 point mutations in and near the conserved fusion loop (called Equad proteins) (9). All samples were positive for DENV IgM (S. Ulbert, unpub. data). This Equad-based ELISA was previously shown to be capable of discriminating DENV from other flaviviruses (9,10). We took the Equad antigens from this ELISA and created a DENV serotyping ELISA. In brief, we titrated serum samples (1:100–1:12,800 in serial 2-fold dilutions) in duplicate on plates coated with DENV-1–4 Equad proteins (Appendix) (10). We defined the endpoint titer for every serotype as the last dilution presenting a signal above the cutoff (Appendix Table), which was calculated as the mean plus 2 times the SD of 15 flavivirus-negative control serum samples (diluted 1:100) acquired from Padova University Hospital, Padova, Italy.
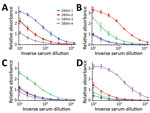
Figure 1. DENV IgM ELISA titers, by serotype, for DENV PCR–positive serum samples from travelers returning to Germany or Italy, 2013–2016. A) DENV-1; B) DENV-2; C) DENV-3; D) DENV-4. Data lines indicate average...
Results showed 1 dominant serotype (Figure 1) for 91.1% of serum samples from returning travelers and 86.5% of serum samples from residents of DENV-endemic countries (Appendix Figures 1, 2). Compared with samples from Vietnam, 15% fewer samples from Sri Lanka had a dominant serotype (Table 1). Serum samples from patients in Sri Lanka cross-reacted only between serotypes 1 and 2 (Appendix Figure 2); however, these samples were collected at a time when the dominant serotype in circulation was switching from DENV-1 to DENV-2 after a DENV-1 outbreak in early 2016 (W. Kumbukgolla, unpub. data). Therefore, this result could be explained by preexisting IgM or, alternatively, by co-infections. However, co-infections were not evident by PCR.
Overall, for 97.1% (102/105) of samples with a dominant serotype, ELISA results corresponded with PCR results; for patients residing in endemic regions, 100% (64/64) of the sample results corresponded, and for returning travelers, 92.7% (38/41) of the sample results corresponded (Table 1). When including the samples for which no dominant serotype was detectable, the overall serotype specificity of the Equad-based ELISA was 85.7% (Table 1); specificity of the ELISA for serum samples from persons in endemic countries was 86.5% and for returning travelers 84.4%. Because of the lower number of dominant serotypes detected, the cohort from Sri Lanka displayed a lower ELISA specificity (81.4%) than did the cohort from Vietnam (95.8%). The serotype results for the PCR-negative group from Vietnam were consistent with the PCR-positive group from Vietnam: ≈80% DENV-1 and ≈20% DENV-2 (Tables 1, 2).

Figure 2. DENV IgM ELISA endpoint titers, by serotype, of paired patient serum samples acquired at 2–4 time points after symptom onset, 2013–2018. A–C) Serum samples from returning travelers with residence in Germany:...
Analysis of paired serum samples suggests that the results of this Equad-based ELISA are consistent over time and with different initial antibody concentrations (Figure 2). The IgM serotype initially identified as the dominant serotype remained the dominant serotype for at least 8 days (and potentially longer) after symptom onset for both returning travelers and patients residing in DENV-endemic countries, many of whom probably had secondary DENV infections (Appendix Figure 3).
Early identification of the infecting DENV serotype can be a critical component of dengue diagnosis that is also essential to pathologic and epidemiologic monitoring of outbreaks. Because of its long persistence in serum, IgM is a preferred diagnostic marker, especially when viral nucleic acids and NS1 are no longer detectable. However, serologic determination of the infecting serotype is challenging. Detection of neutralizing antibodies remains the standard method for DENV identification, but interpretation is complicated by the antigenic sin phenomenon in secondary infections (11). The possibility of using IgM to determine the infecting serotype was attempted previously (12–14), but results were complicated by low specificities, especially for secondary DENV infections. In this study, we analyzed the serotype-specific IgM responses to DENV infections in returning travelers and residents in DENV-endemic regions using recombinant mutated envelope proteins with reduced cross-reactivity (9,10). The ELISA was able to specifically detect the infecting DENV serotype in 84.4% of travelers and 86.5% of residents in DENV-endemic regions. If a dominant serotype was detected, it corresponded to the PCR result in 100% of cases in DENV-endemic regions. The finding that some sample sets did not have a dominant IgM serotype remains to be explained. Factors such as a change in the DENV serotype during an outbreak (as seen in the Sri Lanka cohort) or differing patient exposure histories could be involved. The study included DENV-3–positive serum samples from returning travelers but not from persons residing in endemic regions. However, results obtained for the other serotypes indicate that specificities were similar for samples from returning travelers and inhabitants of endemic areas.
In summary, our results suggest that specific IgM serotyping can be achieved with an ELISA-based format when using as antigens DENV envelope proteins reduced in cross-reactivity. The test can be optimized further by, for example, varying the serum dilutions tested. By using IgM-based serologic tests, which have broad diagnostic windows (15), we can more accurately report epidemiologic outbreak findings.
Dr. Rockstroh is a scientist at the Department of Immunology at the Fraunhofer-Institute for Cell Therapy and Immunology in Leipzig, Germany. Her research interest focus is on the serologic diagnosis of flavivirus infections.
Acknowledgment
Parts of this work were supported by the European Union (European Funds for Regional Development grant 100216415). S.U. and A.R. are on a patent application that includes the proteins described in this study. This does not alter their adherence to policies on sharing data and materials.
References
- Gubler D, Kuno G, Markoff L. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields virology, vol. 1, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 1153–252.
- Nawa M, Yamada KI, Takasaki T, Akatsuka T, Kurane I. Serotype-cross-reactive immunoglobulin M responses in dengue virus infections determined by enzyme-linked immunosorbent assay.Clin Diagn Lab Immunol. 2000;7:774–7.PubMed
Figures
Tables
Suggested citation for this article: Rockstroh A, Barzon L, Kumbukgolla W, Su HX, Lizarazo E, Vincenti-Gonzalez MF, et al. Dengue virus IgM serotyping by ELISA with recombinant mutant envelope proteins. Emerg Infect Dis. 2019 Jan [date cited]. https://doi.org/10.3201/eid2501.180605
Original Publication Date: 11/2/2018





















.png)












No hay comentarios:
Publicar un comentario