
Volume 25, Number 10—October 2019
Research
Sporotrichosis in the Highlands of Madagascar, 2013–20171
Article Metrics
Tahinamandranto Rasamoelina, Danièle Maubon, Onivola Raharolahy, Harinjara Razanakoto, Njary Rakotozandrindrainy, Fetra Angelot Rakotomalala, Sébastien Bailly, Fandresena Sendrasoa, Irina Ranaivo, Malalaniaina Andrianarison, Benja Rakotonirina, Abel Andriantsimahavandy, Fahafahantsoa Rapelanoro Rabenja, Mala Rakoto Andrianarivelo, Lala Soavina Ramarozatovo, and Muriel Cornet
Abstract
Sporotrichosis is a saprozoonotic fungal infection found mostly in tropical and subtropical areas. Few case reports in Madagascar have been published. To document sporotrichosis epidemiology in Madagascar, we conducted a cross-sectional study. During March 2013–June 2017, we recruited from select hospitals in Madagascar patients with chronic cutaneous lesions suggestive of dermatomycosis. Sporotrichosis was diagnosed for 63 (42.5%) of 148 patients. All but 1 patient came from the central highlands, where the prevalence was 0.21 cases/100,000 inhabitants. Frequency was high (64.7%) among patients <18 years of age. Sporotrichosis was diagnosed for 73.8% of patients with arm lesions, 32.3% with leg lesions, and 15.4% with lesions at other sites. Molecular identification identified 53 Sporothrix schenckii isolates. Among the 32 patients who were followed up, response to itraconazole was complete or major for 15 and minor for 17. Overall, endemicity of sporotrichosis in Madagascar was high, concentrated in the highlands.
Sporotrichosis is a chronic fungal infection of humans and animals, found mostly in tropical and subtropical regions. The causal fungi develop in the soil or on plants and infect mammals through wounds, either directly (wounds from spiky plants or thorns) or through contact with contaminated soil or infected animals. Thus, sporotrichosis is a so-called implantation mycosis, affecting principally rural populations, particularly those who work with bare hands and feet (1–3). The disease is caused by a dimorphic fungus of the genus Sporothrix. These fungi display a high degree of genomic diversity, leading to the description of at least 6 cryptic species: S. schenckii, S. brasiliensis, S. globosa, S. luriei, S. mexicana, and S. albicans (formerly S. pallida). S. mexicana and S. albicans are mostly environmental (saprophytic and nonpathogenic) (4–7). The infection generally occurs as a lymphocutaneous form with an ulcerated subcutaneous nodule at the inoculation site and similar secondary lesions arising along the lymphatic route (1,5). Mucosal or primary pulmonary forms are less common (8). Some cases occur as disseminated forms with multiorgan involvement, most notably in HIV-infected persons (9,10).
Sporotrichosis is widespread throughout the world; several areas of known hyperendemicity are Brazil, Mexico, Peru, and China. Outbreaks from various environmental sources, involving thousands of persons, have been reported (1–3,11,12). In Brazil, a large zoonotic outbreak associated with cats is ongoing; it has been suggested that a strain of S. brasiliensis with enhanced virulence is involved (13–15).
In Madagascar, no epidemiologic data are available for evaluation of the sporotrichosis burden. Dermatologists and infectious disease specialists have reported encountering a large number of suspected cases during their routine medical consultations; however, the cases have not been biologically confirmed. In 2007, the dermatology department of Antananarivo University Hospital in the capital of Antananarivo confirmed a series of cases and reported 1 case (16). Since 2013, we conducted a cross-sectional study to document the current epidemiology of this fungal infection in Madagascar. To diagnose, confirm, and identify the fungal species responsible, we used conventional mycology and molecular biology methods, including matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry. We describe the average annual prevalence and clinical presentation of sporotrichosis in Madagascar, along with patient outcomes. We also report the species-level identification, genetic relatedness, and antifungal susceptibility of the clinical isolates.
Study Design and Patient Recruitment
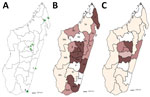
We performed a cross-sectional study of patients with clinically suspected sporotrichosis or another chronic dermatomycosis (i.e., unique or multiple nodular, budding, wart-like, or plaque-like skin lesions following a lymphatic vessel, with or without ulceration, enduring for >1 month). Patients were recruited during March 2013–June 2017 (4 years and 3 months, hereafter referred to as a 4-year period) from the Dermatology Department of the Joseph Raseta Befelatanana University Hospital (CHUJRB) in Antananarivo or during advanced consultation campaigns in regional hospitals (Figure 1, panel A). These campaigns were preceded by announcements made via radio, social media, and posters that invited patients with cutaneous or subcutaneous lesions to come to these consultations. Healthcare providers completed clinical and demographic information forms that asked about the patient’s age, sex, and occupation; the probable area of contamination; anatomic location and appearance of the lesions; and treatments received. We excluded from the study 2 patients for whom this information could not be obtained. The study was approved by the Ethics Committee for Biomedical Research of the Ministry of Public Health of Madagascar (authorization no. 66-MSANP/CE). After sampling, treatment (200 mg/d of itraconazole) was provided free of charge to all recruited patients for at least 2 months.
Statistical Methods
We compared sporotrichosis cases and other nonsporotrichosis cases by using χ2 or Fisher exact tests for qualitative variables and Student t tests for quantitative variables and a χ2 test for trend to compare annual frequencies. We estimated the average annual prevalence as the mean number of cases per year (considering the period as a 4-year period) over the mean number of inhabitants. The mean number of inhabitants was calculated from the available figures in 2013 (obtained from the National Institute of Statistics of Madagascar, ) and adjusted according to the estimate of the World Bank for demographic growth of 2.7% per year (). In our evaluation of the prevalence in the highlands of Madagascar, we excluded the region of Haute Matsiatra because no patients were recruited from there. We analyzed data and generated maps by using EpiInfo version 7.2.2.1 (https://www.cdc.gov/epiinfo/index.html) and RStudio version 1.0.153 ().
Case Definitions
We used a list of clinical, mycologic, histologic, and prognostic criteria to classify cases in this study (Table 1). Cases were identified after a monthly consultation among clinicians of the Department of Dermatology of the CHUJRB and teams of mycologists from the Charles Mérieux Infectiology Center of Antananarivo, Madagascar, and Université Grenoble Alpes, Grenoble, France.
Clinical Samples
After obtaining patient consent, we collected specimens consisting of biopsy material, pus, or flakes of skin from all patients. All samples were sent to the laboratory of the Charles Mérieux Infectiology Center of Antananarivo, where they were processed either immediately or after 24 to 48 hours of storage at 2°–8°C.
Mycologic Analyses
We directly examined the clinical specimens under a microscope (5,11). The samples were used to inoculate Sabouraud medium supplemented with chloramphenicol and incubated at 30°C for 2–3 weeks. For positive cultures, we identified the fungal isolates morphologically, extracted DNA, and froze the culture at −80°C.
Molecular Analyses
We used the QIAamp DNA Blood Mini Kit () according to the manufacturer’s instructions for DNA purification. Colonies and biopsies were crushed before processing. PCR amplification was performed in 2 steps. The first step comprised 2 panfungal PCRs targeting internal transcribed spacer (ITS) regions with the primers ITS1/ITS4 and D1D2 with the primers NL-1/NL-4 and NL-3/NL-4 (17–19). The second step was a specific S. schenckii PCR targeting the topoisomerase II gene with SSHF31/SSHR97 primers (20). We sequenced panfungal PCR products by LGC Genomics GmbH () by using the same primers and aligned the sequences obtained with the reference sequences in the International Society of Human and Animal Mycology (ISHAM) Barcoding Database () (17) for the ITS region and the National Center for Biotechnology Information database for the D1D2 and ITS regions. We constructed the phylogenetic tree by using MEGA7 software ().
MALDI-TOF Mass Spectrometry Analysis
We created a main spectrum profile (MSP) in-house Sporothrix library on the Microflex mass spectrometer, according to the MALDI Biotyper version 1.1 MSP creation protocol (Bruker Daltonicks, ) from a reference strain of S. schenckii (IHEM 3787) and 18 isolates formally identified by DNA sequencing or the specific S. schenckii topoisomerase II PCR. We cultured isolates under 3 conditions: in Sabouraud–chloramphenicol agar for 4–7 days at 30°C, in liquid Sabouraud medium for 2–4 days at 25–30°C with shaking, and on solid peptone dextrose agar (YPD; Sigma Aldrich, ) for 4–5 days at 30°C. This library was validated with the IHEM 3774 reference strain and 35 clinical strains obtained during this study but not used to create the MSPs. We used the ethanol formic acid extraction procedure on YPD subcultured strains in accordance with the MALDI BiotyperIVD protocol version 1.6. We compared the spectra obtained with the Bruker Taxonomy (7,815 entries), Bruker Filamentous Fungi (364 MSP), NIH mold (365 profiles) (21), and our new MSP in-house Sporothrix library, generating identification scores with the following quality criteria: score >2, species-level identification; score <1.7 to <2, genus-level identification; score <1.7, no identification. In addition, we applied an external control by submitting both our MSP in-house Sporothrix and identification spectra to an independent online database, MSI (). This database contains reference spectra for S. schenckii, S. brasiliensis, S. urviconia, S. fungorum, S. globosa, S. humicola, S. inflata, S. insectorum, S. pallida, S. stenoceras, and S. variecibatus (22).
Susceptibility to Antifungal Drugs
To determine the MICs of antifungal agents, we used the Clinical and Laboratory Standards Institute () protocol for filamentous fungi on mycelial strains after subculture at 30°C (23). We tested the following agents at the concentrations indicated: posaconazole and isavuconazole (0.016 to 8 μg/mL), amphotericin B and itraconazole (0.006 to 32 μg/mL), and terbinafine (0.008 to 4 μg/mL). We determined MICs after 72 hours of culture at 30°C with a 100% inhibition endpoint for all drugs except terbinafine, for which the endpoint was 80%, as described by Espinel-Ingroff et al. (24).
Demographic and Clinical Characteristics of the Patients
Total Cohort
During March 2013–June 2017, we recruited 148 patients with chronic cutaneous or subcutaneous lesions. Median patient age was 39 years (interquartile range 22–53 years). Male patients predominated (n = 111, 75%), and the largest number of patients (n = 118, 79.7%) was enrolled by CHUJRB, the permanent recruitment center (Figure 1, panel A, triangle d). An analysis of the geographic origin of recruited patients showed that most (n = 90, 60.8%) came from the highlands, followed by the regions of the northeast (n = 23, 15.5%), east and southeast (n = 16, 10.8%), south and southwest (n = 13, 8.8%), and west (n = 6, 4.1%) (Figure 1, panel B). The largest proportion of recruited patients worked in agriculture (n = 76, 51.3%), followed by the service sector (n = 31, 21%); other patients were students (n = 20, 13.5%), craftsmen (n = 14, 9.5%), or unemployed (n = 7, 4.7%). Lesions were located mostly on the legs (62.8%) and arms (28.3%).
Patients with Sporotrichosis
At the first consultation, 47 of the 148 patients had clinically suspected sporotrichosis. A diagnosis of sporotrichosis was recognized for 63 (42.5%) patients, confirmed for 53 (35.8%), and possible for 10 (6.7%). The frequency of sporotrichosis remained stable from 2013 through 2017 (37.5%–64%, p = 0.16; Table 2). From 2014 through 2016, the mean (+SD) number of annual sporotrichosis cases was 14 (+5.1).
The male sex predominance was less marked among patients with sporotrichosis (n = 44, 69.8%) than among the other patients recruited. This difference, however, was not significant (p = 0.29) (Table 2).
Patients with sporotrichosis were younger than other patients (38 vs. 43 years of age), although this difference was not statistically significant (p = 0.10). Analysis by age group showed that this trend was linked to a high frequency of sporotrichosis in persons <18 years of age (64.7%) compared with the rest of the population (39.7%; p = 0.08).
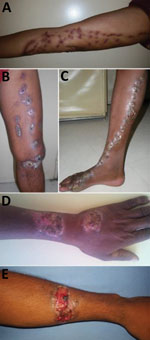
The location of lesions differed significantly between sporotrichosis patients and other patients (Table 2). The principal site affected was the arms (49.2%) for patients with sporotrichosis. Sporotrichosis was diagnosed more frequently for patients with lesions on the arm (73.8%) than for patients with lesions on the leg (32.3%) or other body sites (15.4%) (p<0.0001; Table 2). The sporotrichosis lesions had been present for <1 year for 71% of patients, 1–2 years for 14.5% of patients, and >2 years for 14.5% of patients. The most frequent type of lesion for sporotrichosis patients was lymphocutaneous (69.3%). The other forms were characterized by vegetative or verrucous lesions, infiltrated plaques, or tuberous lesions (Figure 2). We observed no fixed cutaneous forms or extracutaneous forms.
Sporotrichosis patients were predominantly farmers (52.4%), but a large number were craftsmen and tradesmen (Table 2). Sporotrichosis was diagnosed for 71.4% of the craftsmen and tradesmen and 39% of patients in other professions grouped together (p = 0.04).
Prevalence and Geographic Distribution of Sporotrichosis
We determined the geographic origin of patients with sporotrichosis, corresponding to the presumed origin of contamination (Figure 1, panel C). The concentration of sporotrichosis cases in the highlands was very high; almost all patients with sporotrichosis (n = 62, 98.4%) originated from these areas. The frequency of sporotrichosis cases was much higher in the highlands (62/90, 68.9%) than on the rest of the island (1/58, 1.7%; p<0.0001). In all highland areas, the frequency of sporotrichosis was similar and very high (66.6%–100%; p = 1.00) (Table 2).
The average annual prevalence of sporotrichosis on the high plateaus of the Analamanga, Amoron’i Mania, Bongolava, Itasy, and Vakinankaratra regions was evaluated at 0.21 cases/100,000 inhabitants. Prevalence was highest in the Analamanga (0.27/100,000 inhabitants) and Amoron’i Mania (0.25/100,000 inhabitants) regions (Table 3; Figure 1, panel C).
Mycologic Results
We collected 192 samples from the 148 patients. Direct examination yielded negative results for all cases, and we were unable to perform histologic examinations (Appendix 1).
Culture Results
We obtained 172 cultures, including 72 established with the samples of the 63 sporotrichosis patients. Overall, macroscopic and microscopic examination exhibited morphologic features consistent with Sporothrix spp. for 90.2% (65/72) of the cultures.
Molecular Results
Sensitivities for the 2 panfungal PCRs, for D1D2 and ITS, were lower for clinical specimens than for cultures, and the ITS PCR was less sensitive than the D1D2 PCR for clinical specimens and cultures (Appendix 2 Figure 1). The specific S. schenckii topoisomerase II PCR was unable to confirm identification for any of the clinical specimens, whereas its sensitivity for cultures was 89.2% (58/65), with features suggestive of Sporothrix spp.
The alignment of the ITS sequences from cultures identified 13 isolates as S. schenckii, according to data from the ISHAM database (Appendix 1 Table 1) (17). The phylogenetic analysis showed that the isolates from patients with sporotrichosis in our study were grouped in the S. schenckii clade, together with clinical strains from other regions of the world (Figure 3; Appendix 2 Figure 2). Our results confirm that ITS sequencing is suitable for separating the cryptic species of clinical importance from the strictly environmental ones (25).
The MSP in-house Sporothrix library performed well for S. schenckii identification; the score for 87.9% (29/33) of the strains was >2 and for 4 strains was >1.8. Unfortunately, 2 strains were contaminated by Candida spp. and could not be identified. For sporotrichosis identification, in-house MSPs were systematically the first choice in the list of MSPs, confirming their superiority for identification at the species level over the 2 MSPs in the Bruker database and the 1 MSP in the NIH database. Comparison of the spectra obtained by using the MSP in-house Sporothrix library with those obtained from identification to the external MSI platform indicated that the most likely identification was S. schenckii (Appendix 1 Table 2). Percentages of similarities were consistent with accurate identification to the species level (>20%) for 94.1% (48/51) (Appendix 1 Table 1); only 3 strains were not formally identified.
Taking together all results of the molecular analyses, we identified 53 S. schenckii strains: 51 by MALDI-TOF mass spectrometry, of which 46 were identified also by the specific S. schenckii topoisomerase II PCR and 13 were identified also by ITS sequencing. The 2 contaminated isolates not identified by MALDI-TOF mass spectrometry were confirmed by the specific S. schenckii topoisomerase II PCR.
Susceptibility of Sporothrix schenckii to Antifungal Drugs and Patient Outcomes
A total of 46 S. schenckii isolates were culturable after thawing for MIC determination. We determined MICs and their geometric means for the 5 antifungal drugs tested (Table 4). Overall, of the S. schenckii isolates, the terbinafine MIC was low (≤0.25 μg/mL) for 82.3% (38/46), the itraconazole MIC was ≤1 μg/mL for 74% (34/46), and the posaconazole MIC was ≤1 μg/mL for 56.5% (26/46). However, 65% of strains had MICs ≥4 μg/mL for isavuconazole.
All 63 patients received treatment, but only 32 were monitored for >2 months after treatment began (23 with lymphocutaneous disease and 9 with minor forms of disease). Among those patients, the response was complete or major for 15 (47%) after 4–7 months of treatment and minor for 17 (53%) after prolonged (9 months) treatment. The remaining 31 patients received treatment for 2 months and then did not return for follow-up visits.
This study provides recent epidemiologic data for sporotrichosis in Madagascar. We detected numerous cases and substantial endemicity despite previous reports of only sporadic cases or small series (16,26). We estimated an average annual prevalence in the highlands of 0.21 cases/100,000 inhabitants; 98% of the cases were concentrated in that area. Among sporotrichosis patients in Madagascar, we highlight the high infection risk for young persons (<18 years of age) and the particularly high frequency of lesions on the arms. On the basis of this study, we were able to develop and routinely implement molecular analyses in Madagascar, enabling positive identification of S. schenckii for all confirmed cases.
The high estimated prevalence (0.27 and 0.25/100,000 inhabitants) in 2 highland regions (Analamanga and Amoron’i Mania) reveals the high transmission rates in this part of the island. To date, the only published series of sporotrichosis cases in Madagascar have described disease-endemic foci in the highlands, particularly in the Analamanga region (16), but the concentration of cases in the highlands that we observed was unexpected and striking. This almost exclusive distribution may be the result of climate conditions in this region, which differ from those on the rest of the island. This region has a tropical climate, with a mean temperature of 19.5°C and substantial rainfall, which probably favors development of fungi on plants and in the soil. A phylogeographic study focusing strictly on S. schenckii showed that this species was present in temperate (United States), hot and humid (South Africa, Australia, Colombia, and Venezuela), hot and dry (Australia and Uruguay), cool and dry (Peru and South Africa), and cool and humid (Uruguay) zones (27). Findings of that study therefore seem to go against the notion of a single climatic factor. The frequency of sporotrichosis in some areas of the island to the west and southwest are unknown because these areas were not investigated; thus, sporotrichosis may not be totally distributed in the highlands. In addition, some cases could have been missed because of our mode of study recruitment, the low incomes of people living in remote areas, and the limited development of the healthcare system. The concentration of sporotrichosis in the highlands is probably not the result of better access to healthcare facilities because patients with other diagnoses most frequently do not live in the highlands (p<0.0001) (Table 2). However, better access to medical care in the highlands does partly explain the sporotrichosis diagnoses made relatively early in the course of disease (71% in the first year after onset).
Patients found it difficult to remember when their lesions had appeared and to associate them with a particular injury or activity; however, our survey revealed considerable involvement in rural activities: farming (rice, cassava, corn), logging, trade, and craftsmanship. Not only artisans are exposed to contamination through manual work; tradespeople are also exposed because they practice activities other than selling for living. Contamination by activities associated with the manual production of charcoal and cutting wood for cooking and heating seems likely on the basis of the predominance of arm lesions, the concentration of the disease in the coldest region of the island, and the high frequency of infections among children (who practice these activities). In northeastern China, a risk associated with the use of wood or other fuel has been proposed as an explanation for transmission patterns (28); the authors of that study thought that contamination occurred via maize stalks (where S. globosa has been found) used for heating and cooking. They hypothesized that the fermentation of the plants promotes the development of yeast forms of the fungus, increasing the risk for contamination during transport and storage at home.
Other possible sources of contamination, such as soil or decaying plant material, are also possible in Madagascar. Neither we nor J.F. Carod et al. (16) observed any cases of zoonotic transmission; the identification of S. schenckii alone confirms the hypothesis of contamination by soil and plants.
The molecular methods that we developed in this study, including MALDI-TOF mass spectrometry, made it possible to confirm cases and to identify the species responsible (21,29). We found that it was easier to amplify the D1D2 domain (LSU) and that the amplicons obtained were easier to analyze by sequencing than were those of the ITS domain. However, the availability of a database with many verified ITS sequences and the more polymorphic and discriminant nature of these sequences makes them more suitable for cryptic species identification and phylogenetic analysis (17,25).
We added a rapid and inexpensive mass spectrometry identification approach to the molecular tools for identifying S. schenckii to the cryptic species level. Our results obtained by using a Bruker instrument confirm previous analyses performed with a Shimadzu instrument (29). The MSP in-house Sporothrix library yielded better S. schenckii identification scores than did the 3 preexisting MSPs. The excellent identification scores and the external validation with another mass spectrometry platform showed that S. schenckii identification at the species level with MALDI-TOF mass spectrometry is accurate and adapted for routine diagnoses in clinical laboratories.
In conclusion, our study reveals substantial endemicity of sporotrichosis in Madagascar. Sporotrichosis was particularly concentrated in the highlands, which have climate, vegetation, and lifestyle conditions that favor the development and transmission of the fungus. Using molecular methods and MALDI-TOF mass spectrometry, we were able to identify S. schenckii as the species responsible for sporotrichosis in Madagascar. Despite its high frequency, sporotrichosis remains neglected in Madagascar.
Dr. Rasamoelina is a scientist in the Centre d’Infectiologie Charles Mérieux in Antananarivo, Madagascar. His research interest is the laboratory diagnosis of tropical mycoses.
Acknowledgments
This work was supported by the Fondation Mérieux, Lyon, France; the Institut de Recherche pour le Développement; the Société Française de Mycologie Médicale; and Campus France.
M.C. received research grants from Pfizer and travel grants from Basilea, Gilead, MSD, and Pfizer.
References
- Barros MBL, de Almeida Paes R, Schubach AO. Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev. 2011;24:633–54.
- Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. Neglected endemic mycoses. Lancet Infect Dis. 2017;17:e367–77.
- Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53:3–14.
- Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198–206.
- Buot G, Develoux M, Hennequin C. Sporotrichose. Encycl Méd Chir. 2017;14:1–10.
- Mora-Montes HM, Dantas AS, Trujillo-Esquivel E, de Souza Baptista AR, Lopes-Bezerra LM. Current progress in the biology of members of the Sporothrix schenckii complex following the genomic era. FEMS Yeast Res. 2015;15:
fov065 . - Lopes-Bezerra LM, Mora-Montes HM, Zhang Y, Nino-Vega G, Rodrigues AM, de Camargo ZP, et al. Sporotrichosis between 1898 and 2017: The evolution of knowledge on a changeable disease and on emerging etiological agents. Med Mycol. 2018;56(suppl_1):126–43.
- Rojas FD, Fernández MS, Lucchelli JM, Lombardi D, Malet J, Vetrisano ME, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119–23.
- Freitas DF, Lima MA, de Almeida-Paes R, Lamas CC, do Valle AC, Oliveira MM, et al. Sporotrichosis in the central nervous system caused by Sporothrix brasiliensis. Clin Infect Dis. 2015;61:663–4.
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, Basílio-de-Oliveira RP, de Araujo LF, Martins CJ, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140.
- Rasamoelina T, Raharolahy O, Rakotozandrindrainy N, Ranaivo I, Andrianarison M, Rakotonirina B, et al. Chromoblastomycosis and sporotrichosis, two endemic but neglected fungal infections in Madagascar. J Mycol Med. 2017;27:312–24.
- Ramírez Soto MC. Sporotrichosis among children of a hyperendemic area in Peru: an 8-year retrospective study. Int J Dermatol. 2017;56:868–72.
- Rodrigues AM, de Melo Teixeira M, de Hoog GS, Schubach TM, Pereira SA, Fernandes GF, et al. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis. 2013;7:
e2281 . - Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:
e1005638 . - Gremião ID, Miranda LH, Reis EG, Rodrigues AM, Pereira SA. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathog. 2017;13:
e1006077 . - Carod JF, Ramarozatovo L, Randrianasolo P, Ratsima E, Randrianirina F, Rapelanoro FR. [Cutaneous sporotricosis in a Malagasy patient] [in French]. Med Trop (Mars). 2007;67:18.
- Irinyi L, Lackner M, de Hoog GS, Meyer W. DNA barcoding of fungi causing infections in humans and animals. Fungal Biol. 2016;120:125–36.
- Kurtzman CP, Robnett CJ. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol. 1997;35:1216–23.
- Abliz P, Fukushima K, Takizawa K, Nishimura K. Identification of pathogenic dematiaceous fungi and related taxa based on large subunit ribosomal DNA D1/D2 domain sequence analysis. FEMS Immunol Med Microbiol. 2004;40:41–9.
- Kanbe T, Natsume L, Goto I, Kawasaki M, Mochizuki T, Ishizaki H, et al. Rapid and specific identification of Sporothrix schenckii by PCR targeting the DNA topoisomerase II gene. J Dermatol Sci. 2005;38:99–106.
- Lau AF, Drake SK, Calhoun LB, Henderson CM, Zelazny AM. Development of a clinically comprehensive database and a simple procedure for identification of molds from solid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2013;51:828–34.
- Normand AC, Becker P, Gabriel F, Cassagne C, Accoceberry I, Gari-Toussaint M, et al. Validation of a new Web application for identification of fungi by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2017;55:2661–70.
- Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard (document M38–A2). Wayne (PA): The Institute; 2008.
- Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, Brilhante RSN, Chakrabarti A, Chowdhary A, et al. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob Agents Chemother. 2017;61:e01057–17.
- Zhou X, Rodrigues AM, Feng P, de Hoog GS. Global ITS diversity in the Sporothrix schenckii complex. Fungal Divers. 2014;66:153–65.
- Rapelanoro-Rabenja F, Ralandison S, Ramarozatovo L, Randrianasolo F, Ratrimoarivony C. Sporotrichose à Madagascar: une pathologie méconnue. N. 2007;26:10.
- Zhang Y, Hagen F, Stielow B, Rodrigues AM, Samerpitak K, Zhou X, et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia. 2015;35:1–20.
- Li S, Cui Y, Yao L, Song Y. Sporothrix globosa causing sporotrichosis in Jilin Province (Northeast of China): prevalence, molecular characterization, and antifungal susceptibility. Abstract presented at the 20th Congress of the International Society for Human and Animal Mycology; Amsterdam, the Netherlands; 2018 Jun 30–Jul 4. Oxford: Oxford University Press; 2018. p. S19.
- Oliveira MM, Santos C, Sampaio P, Romeo O, Almeida-Paes R, Pais C, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102–10.
Figures
Tables
Cite This ArticleOriginal Publication Date: 9/5/2019
1Preliminary results from this study were presented at the 20th ISHAM Conference; June 29–July 5, 2018; Amsterdam, the Netherlands (abstract no. S1.4d).






















.png)












No hay comentarios:
Publicar un comentario