
Volume 25, Number 10—October 2019
Dispatch
Plasmodium cynomolgi as Cause of Malaria in Tourist to Southeast Asia, 2018
On This Page
Downloads
Article Metrics
Gitte N. Hartmeyer , Christen R. Stensvold, Thilde Fabricius, Ea S. Marmolin, Silje V. Hoegh, Henrik V. Nielsen, Michael Kemp, and Lasse S. Vestergaard
, Christen R. Stensvold, Thilde Fabricius, Ea S. Marmolin, Silje V. Hoegh, Henrik V. Nielsen, Michael Kemp, and Lasse S. Vestergaard
Abstract
We report human infection with simian Plasmodium cynomolgi in a tourist from Denmark who had visited forested areas in peninsular Malaysia and Thailand in August and September 2018. Because P. cynomolgi may go unnoticed by standard malaria diagnostics, this malaria species may be more common in humans than was previously thought.
Despite marked reductions in the global disease burden, malaria remains a serious threat to persons living in or visiting areas to which it is endemic (1). Traditionally, 4 species of Plasmodium parasites (P. falciparum, P. vivax, P. ovale, and P. malariae) have been considered to cause natural human malaria; however, several simian Plasmodium species have also been found to infect humans (2). P. knowlesi, a parasite of forest macaques in Southeast Asia, is regularly detected in human malaria cases, including cases involving tourists (3). Because of morphological similarity, P. knowlesi has been widely misdiagnosed as P. malariae or P. falciparum by microscopy. In Brazil, P. simium was initially identified as P. vivax during outbreaks in 2015 and 2016 (4), highlighting the need for better methods for accurate identification.
In 2014, another simian Plasmodium species, P. cynomolgi, was reported to have naturally infected an adult patient (5). Until then, P. cynomolgi was known as a human parasite only from experimental studies. In addition to fever, clinical symptoms in humans comprise cephalgia, anorexia, myalgia, and nausea; the prepatent period is 7–16 days and the incubation period is ≈15–20 days, with some variation between different strains of P. cynomolgi (2,6).
P. cynomolgi is found in long-tailed macaques across Southeast Asia, often concomitant with other simian malaria parasites such as P. inui, P. coatneyi, or P. fieldi (7). A recent vector survey in Vietnam demonstrated the presence of P. cynomolgi among other human and nonhuman primate Plasmodium spp. parasites in Anopheles dirus, an important local malaria vector (8). Asymptomatic carriage of P. cynomolgi was recently reported in village residents in Cambodia (9). We report a travel-related case of malaria caused by P. cynomolgi in a tourist from Denmark who had traveled to forested areas in peninsular Malaysia and Thailand.
A 37-year-old woman from Denmark with no underlying conditions and no previous history of malaria traveled with her husband and children for 6 weeks in various parts of peninsular Malaysia and Thailand in 2018. None of them took malaria chemoprophylaxis; however, they used mosquito repellents and mosquito nets.
The family traveled by air to Singapore in mid-August 2018 and traveled by bus to Kuala Lumpur, Malaysia. From there, they traveled by air to Kota Bharu on the east coast of peninsular Malaysia and sailed to Perhentian Island, staying for 4 days in a beach cottage, with day trips into the nearby forest. In late August, they returned to Kuala Lumpur and traveled by air to Chiang Mai, Thailand, from which they visited remote mountain villages, hiked through the forest, and stayed overnight in local villages. In early September they traveled by air to Bangkok and traveled onward by train to Khao Sok National Park, Surat Thani Province, where they stayed in treehouses in the jungle for 4 nights. In mid-September they traveled by car and ferry to the island Koh Phangan, where they stayed in beach houses for a week. They then sailed to the island Koh Samui for another week of beach holiday before returning to Denmark.
The patient noted numerous macaque monkeys during the jungle visit in Khao Sok, but not in the other areas. She also reported receiving several mosquito bites while in Khao Sok, despite the use of preventive measures.
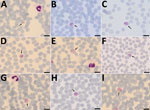
The day before returning to Denmark, the patient experienced muscle pain, general malaise, fever, headache, and abdominal pain. Symptoms gradually declined after 6–8 hours but returned after 12 hours. This cyclic pattern repeated 5–6 times. On the day after her return, she was admitted to the local hospital for fever (39.0°C) and suspected malaria; circulatory and respiratory systems were stable. Chest radiograph results were normal. Blood examination showed slightly elevated C-reactive protein (39 mg/L), leukocyte count within reference range (6.3 × 109 cells/L), slightly elevated S-alanine aminotransferase (S-ALAT, 55 IU/L), and thrombocytopenia (104 × 109 platelets/L); platelet levels on the next day further dropped to 96 × 109 /L. Results of initial malaria testing using a malaria rapid diagnostic test (RDT) (First Response Malaria Ag Combo Test, Premier Medical Corporation Ltd., ) and malaria microscopy were negative. Because her symptoms persisted, we tested an EDTA-blood sample by loop-mediated isothermal amplification (Illumigene Malaria; Meridian Bioscience, ); results were positive for Plasmodium spp. Repeated microscopic examination of Giemsa-stained thin blood smear (10%, pH 7.2 for 15 min) revealed low-grade parasitemia. We observed different parasite stages that resembled P. vivax but with slightly different morphologic features (Figure 1) (2).
We referred the patient to a tertiary hospital for treatment and follow-up. Repeated LAMP was positive for Plasmodium DNA, whereas the rapid test was again negative. In-house real-time PCRs were positive for Plasmodium (10) but negative for P. falciparum (11), P. vivax (11,12), P. ovale (13), P. malariae (13), and P. knowlesi (14). A blood sample was analyzed at the National Reference Parasitology Laboratory by PCR using genus-specific primers (Plasmo F 5′-TTGYCTAAAATACTTCCATTAATCAAGAACG-3′ and Plasmo R 5′-TTTGATTTCTCATAAGGYACTGAAGG-3′) and a next-generation sequencing–based (NGS) microbiota assay, described previously (15). Sanger sequencing of the genus-specific PCR product revealed P. cynomologi. The assay identified 2 stage-specific types of nuclear small subunit (SSU) rRNA genes (16), both belonging to P. cynomolgi. Clone 1 exhibited 99.51% similarity to GenBank accession no. AB287289 (asexual [A]–type SSU rDNA), and clone 2 had 100% similarity to GenBank accession no. AB287288 (sporozoite [S]–type SSU rDNA), both of which were isolates identified in a long-tailed macaque in Southeast Asia (Figure 2).
The patient received atovaquone/proguanil (1,000/400 mg/d for 4 d), followed by primaquine (26.4 mg/d for 14 d). Symptoms resolved on the second day of treatment, and the patient was discharged for outpatient follow-up. Within a week, platelet count normalized, and S-ALAT further increased to 135 U/L. Results of malaria microscopies repeated on days 9 and 37 of treatment were negative. The patient fully recovered.
A short-term traveler contracted P. cynomolgi malaria during a trip to Southeast Asia. Exactly where the patient became infected is not known. The presence of nocturnal mosquitoes and macaques makes Khao Sok National Park in Thailand a likely site of infection. The time interval of 17 days between her arrival in Khao Sok and the onset of symptoms matches reported incubation periods for human P. cynomolgi infections (6).
Surat Thani Province in Thailand is located ≈800 km north of Hulu Terengganu in peninsular Malaysia, where a natural human P. cynomolgi infection was only recently reported in a local resident (5). Long-tailed macaques are present in both of these areas, but not around Chiang Mai (3).
We obtained species identification by DNA sequencing only after negative species-specific real-time PCR. Given the challenge of diagnosing P. cynomolgi and the widespread occurrence of its natural host across Southeast Asia, it is likely that this simian Plasmodium sp. is underdiagnosed in both residents and visiting travelers.
Urban development into forested areas leads to closer coexistence of human and monkey. The number of cases in which malaria is transmitted from monkeys to humans may therefore increase. Advanced detection and identification techniques may improve knowledge of the epidemiology of simian malaria in humans.
Dr. Hartmeyer is a consultant in clinical microbiology at Odense University Hospital, Denmark. Her research interests focus on clinical parasitology and epidemiology.
References
- World Health Organization. World malaria report. 2018 [cited 2019 Jan 16].
- Coatney GR, Collins WE, Warren M, Contacos PG. The primate malarias. Atlanta: Centers for Disease Control and Prevention; 2003 [cited 2019 Aug 6].
- Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013;26:165–84.
- Brasil P, Zalis MG, de Pina-Costa A, Siqueira AM, Júnior CB, Silva S, et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: a molecular epidemiological investigation. Lancet Glob Health. 2017;5:e1038–46.
- Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J. 2014;13:68.
- Contacos PG, Elder HA, Coatney GR, Genther C. Man to man transfer of two strains of Plasmodium cynomolgi by mosquito bite. Am J Trop Med Hyg. 1962;11:186–93.
- Zhang X, Kadir KA, Quintanilla-Zariñan LF, Villano J, Houghton P, Du H, et al. Distribution and prevalence of malaria parasites among long-tailed macaques (Macaca fascicularis) in regional populations across Southeast Asia. Malar J. 2016 Sep 2;15(1) [cited 2019 Feb 20].
- Chinh VD, Masuda G, Hung VV, Takagi H, Kawai S, Annoura T, et al. Prevalence of human and non-human primate Plasmodium parasites in anopheline mosquitoes: a cross-sectional epidemiological study in Southern Vietnam. Trop Med Health. 2019;47:9.
- Imwong M, Madmanee W, Suwannasin K, Kunasol C, Peto TJ, Tripura R, et al. Asymptomatic natural human infections with the simian malaria parasites Plasmodium cynomolgi and Plasmodium knowlesi. J Infect Dis. 2019;219:695–702.
- Kamau E, Alemayehu S, Feghali KC, Saunders D, Ockenhouse CF. Multiplex qPCR for detection and absolute quantification of malaria. PLoS One. 2013;8:
e71539 . - Perandin F, Manca N, Piccolo G, Calderaro A, Galati L, Ricci L, et al. Identification of Plasmodium falciparum, P. vivax, P. ovale and P. malariae and detection of mixed infection in patients with imported malaria in Italy. New Microbiol. 2003;26:91–100.
- Rougemont M, Van Saanen M, Sahli R, Hinrikson HP, Bille J, Jaton K. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol. 2004;42:5636–43.
- Veron V, Simon S, Carme B. Multiplex real-time PCR detection of P. falciparum, P. vivax and P. malariae in human blood samples. Exp Parasitol. 2009;121:346–51.
- Divis PC, Shokoples SE, Singh B, Yanow SK. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar J. 2010;9:344.
- Krogsgaard LR, Andersen LO, Johannesen TB, Engsbro AL, Stensvold CR, Nielsen HV, et al. Characteristics of the bacterial microbiome in association with common intestinal parasites in irritable bowel syndrome. Clin Transl Gastroenterol. 2018;9:161.
- Gunderson JH, Sogin ML, Wollett G, Hollingdale M, de la Cruz VF, Waters AP, et al. Structurally distinct, stage-specific ribosomes occur in Plasmodium. Science. 1987;238:933–7.
Figures
Cite This ArticleOriginal Publication Date: 9/4/2019






















.png)












No hay comentarios:
Publicar un comentario