Volume 25, Number 7—July 2019
Dispatch
Diagnosis of Chagasic Encephalitis by Sequencing of 28S rRNA Gene
On This Page
Downloads
Article Metrics
Ashrit Multani , Aabed Meer, Darvin S. Smith, Malika N. Kheraj, Edward D. Plowey, and Brian G. Blackburn
, Aabed Meer, Darvin S. Smith, Malika N. Kheraj, Edward D. Plowey, and Brian G. Blackburn
Abstract
We report a case of chagasic encephalitis diagnosed by 28S rRNA sequencing. The diagnosis of chagasic encephalitis is challenging, given the broad differential diagnosis for central nervous system lesions in immunocompromised patients and low sensitivity of traditional diagnostics. Sequencing should be part of the diagnostic armamentarium for potential chagasic encephalitis.
Chagasic encephalitis is a rare disease in the United States. We report a case of chagasic encephalitis in an HIV-infected man. This case was diagnosed by sequencing of the parasite 28S rRNA gene.
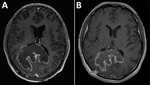
The patient was a 31-year-old HIV-infected man who had fevers, headaches, and ataxia for 3 weeks. He had lived in El Salvador until moving to the United States 6 years earlier. His neurologic symptoms persisted, and he was hospitalized after cranial computed tomography (CT) showed a 6-cm, heterogeneous, centrally necrotic mass in the corpus callosum. At admission, he was afebrile, oriented only to self, and had slow movements.
Testing showed a leukocyte count of 3,500 cells/μL, hemoglobin level of 12.4 g/dL, CD4 cell count of 60 cells/μL, HIV viral load of 409,302 copies/mL, and a positive result for serum Toxoplasma gondii IgG. Chest radiograph results were unremarkable. Magnetic resonance imaging (MRI) of the brain (Figure 1, panel A) showed an 8.1 × 7.3 cm heterogeneous mass centered within the corpus callosum and parietal–occipital subcortical white matter. Diffusion restriction was identified mostly within peripheral portions of the lesion. Administration of gadolinium showed heterogeneous peripheral enhancement and central necrotic change. Additional foci of abnormal fluid-attenuated inversion recovery signal and enhancement were noted within posterior fossa and supratentorial parenchyma. On the basis of these findings, radiologically favored diagnoses included lymphoma, glioblastoma, or tumefactive multiple sclerosis. Infection was believed less likely, given the absence of prominent central diffusion restriction.
Urgent MRI-directed stereotactic biopsy of the brain was performed. Cytologic smear preparations showed an intraoperative pathological impression of toxoplasmosis on the basis of identification of protozoal organisms. The patient was given trimethoprim/sulfamethoxazole for possible toxoplasmic encephalitis while we awaited procurement of pyrimethamine/sulfadiazine.
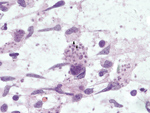
Subsequent review of permanent pathologic sections showed necrotizing encephalitis and abundant amastigotes with prominent kinetoplasts in astrocytes and macrophages (Figure 2). Immunostaining for Toxoplasma spp. was negative. Sequencing of the internal transcribed spacer 2 and D2 regions of the 28S rRNA gene in paraffin-embedded tissue identified the organism as Trypanosoma cruzi (Figure 3) (1,2). A T. cruzi IgG test result was subsequently positive; results of peripheral blood smear examination result were negative for circulating trypomastigotes.
Trimethoprim/sulfamethoxazole was decreased to prophylactic dosing, and benznidazole (2.5 mg/kg 2×/d) was given after receipt of this drug from the Centers for Disease Control and Prevention (Atlanta, GA, USA) Drug Service 6 days after admission. Treatment with lamivudine, zidovudine, and nevirapine was begun 1 week later.
The patient’s course was complicated by leukopenia requiring benznidazole treatment interruption and replacement of zidovudine with abacavir. He completed 60 days of benznidazole therapy over a 3-month period but did not receive secondary prophylaxis for T. cruzi. Three months after initial presentation, his HIV viral load was suppressed, his CD4 count had increased sustainably to >200 cells/μL, he was symptomatically and radiologically (Figure 1, panel B) improved, and he returned to work.






















.png)












No hay comentarios:
Publicar un comentario