
Using PODXL as a Prognostic Biomarker for Colorectal and Urothelial Cancer
Cancer
Cancer is a major global health issue. With the increase in the life expectancy of the world population, there is no reason to expect a decline in cancer incidence in the near future.
Usually, cancer is denoted as a singular disease; however, in reality, it is a multitude of diseases. Although this insight has been gained over the years, thus far, many patients have not been able to receive optimal treatment for their disease. If cancer patients have to receive a more individualized treatment, innovative and optimized ways to stratify patients are required.
The classical, pathology-based prognostic factors like stage and grade of the tumor are not adequate for precise estimation of patient prognosis. Supplementary information from immunopathology biomarkers look promising in considerably improving this estimation, eventually resulting in a more individualized treatment, thereby preventing under- or over-treatment of patients.
PODXL
Podocalyxin (PODXL) is a transmembrane protein that plays a role in cell-cell interaction. In the case of normal tissues, the protein is expressed in the kidney glomerular podocytes, where it has a vital role in maintaining filtration pathways, as well as in endothelial cells in blood vessels. In various types of cancer such as prostate, breast, and testicular cancer, PODXL tends to be overexpressed. On the Human Protein Atlas (proteinatlas.org),1,2,3,4 the PODXL protein was recognized as a prospectively intriguing testicular cancer biomarker, and later, it was identified to be a prognostic biomarker in both colorectal5 and urothelial cancer.6
PODXL in Colorectal Cancer
One of the most common types of cancer is colorectal cancer. Every year, about one million new cases of colorectal cancer are detected, and worldwide, around 600,000 deaths can be attributed to this disease. At present, the only curative treatment for colorectal cancer is surgery; however, adjuvant treatment might considerably improve patient survival. However, if the adjuvant treatment has to be successful, the precise identification of patients who can benefit from treatment is vital.
Currently, adjuvant treatment is recommended for patients with stage III and high-risk stage II disease. Hence, for patients suffering from stage II colorectal cancer, it is crucial to identify biomarkers with the ability to differentiate high-risk disease from low-risk disease.
Immunohistochemistry (IHC) has been used to analyze PODXL protein expression in three distinct colorectal cancer patient cohorts.5 It was demonstrated that high membranous PODXL expression in the tumor is an independent predictor of poor prognosis in all three cohorts. Figure 1 illustrates the IHC stainings of membranous versus no or non-membranous positivity using a monoclonal Anti-PODXL antibody. In none of the cohorts was there a link between PODXL expression and gender, age at diagnosis, or tumor location.
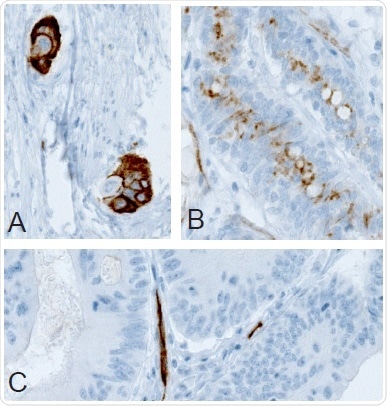
Figure 1. (A) Membranous, (B) non-membranous/cytoplasmic, and (C) absence of immunoreactivity in colorectal tumor samples following IHC staining with Anti-PODXL (AMAb90667) antibody.
The survival of colorectal cancer patients based on membranous PODXL expression in the tumors is illustrated in Figure 2A. In all the cohorts, reduced patient survival was linked to a high membranous PODXL expression. It was observed that there was a similarity in colorectal cancer-specific survival (CCSS) between patients with tumors that expressed high membranous levels of PODXL treated with adjuvant chemotherapy (CT) and patients with PODXL-low tumors. As shown in Figure 2B, the CCSS of untreated patients with PODXL-high tumors was shorter than all the other patient groups (Figure 2B).
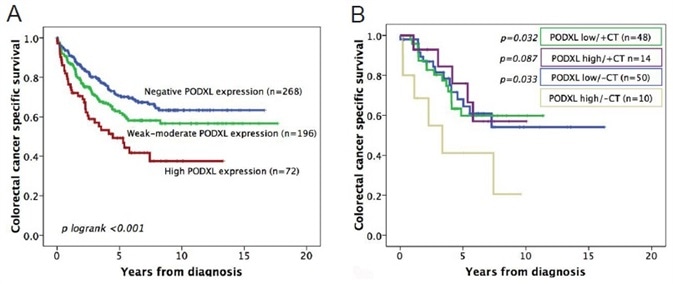
Figure 2. (A) Kaplan-Meier analysis of colorectal cancer-specific survival of patients with tumors expressing no, weak/moderate, or high levels of PODXL protein. (B) Kaplan-Meier analysis of colorectal cancer-specific survival, where patients are divided into groups according to whether they received adjuvant chemotherapy (CT) or not, as well as the level of tumor PODXL expression (high or low).
These outcomes indicate that adjuvant chemotherapy would be beneficial for patients with tumors that express high membranous levels of PODXL.
PODXL in Urothelial Cancer
In the case of urothelial cancer, a major clinical problem is the identification of high-risk patients from those diagnosed with T1 disease (in which the tumor has invaded connective tissue but not the muscle). Although the prognosis is often good for such stage T1 patients, almost one-third will still ultimately need cystectomy following the failure of other therapies. Early-stage identification of these patients would relate to considerable improvement for patients and also the society.
The health care cost per patient for the treatment of urothelial cancer is high due to high recurrence rates and the need for invasive monitoring of the disease. In the case of patients with muscle-invasive disease, prognostic markers that could help in choosing the treatment and/or treatment intensity would also relate to improvement in patient care.
Both the Cox univariable analysis and the multivariable analysis adjusted for gender, age, T-stage, and grade confirmed the link between membranous PODXL expression and reduced survival. Analysis of the membranous PODXL expression in the subgroup of patients with stage Ta (non-invasive) and T1 tumors revealed that membranous PODXL expression was linked with heightened risk of disease progression and also increased risk of death from disease. An example of membranous PODXL expression from a urothelial carcinoma sample using IHC is illustrated in Figure 3.
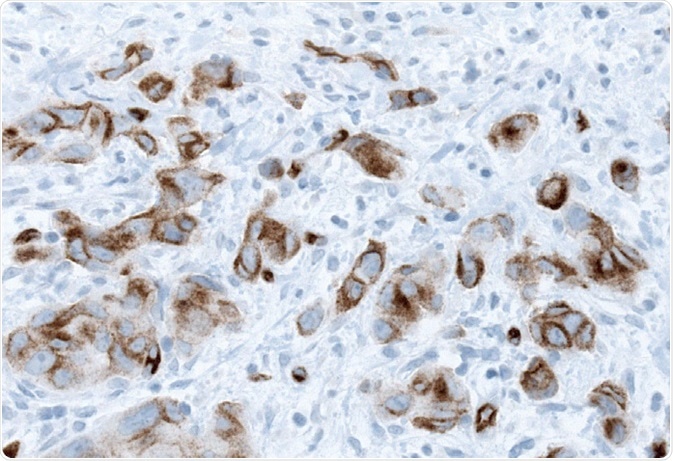
Figure 3. Membranous PODXL expression in urothelial cancer obtained by IHC analysis using the Anti-PODXL antibody AMAb90643.
IHC has been used to analyze PODXL protein expression in two different urothelial cancer patient cohorts (Cohort I, n = 110 and Cohort II, n = 344, respectively). As shown in Figure 4A and 4B, in comparison with no or non-membranous expression, membranous PODXL expression in the tumor was found to be linked with a reduced five-year overall survival in both patient cohorts.
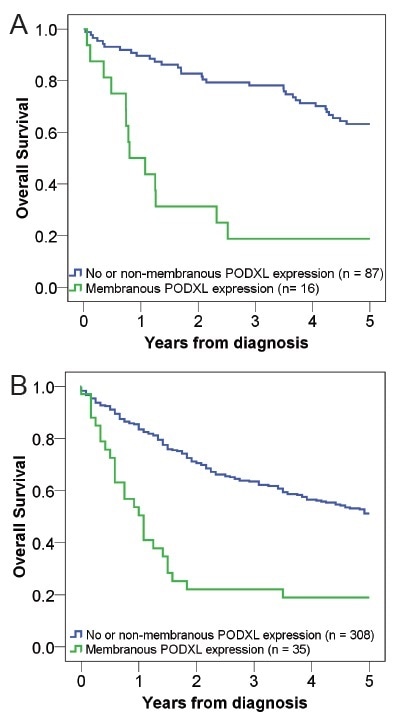
Figure 4. Kaplan–Meier estimates of five-year Overall Survival (OS) according to PODXL expression in (A) Cohort I and (B) Cohort II.
PrecisA Monoclonals Against PODXL
Atlas Antibodies (Stockholm, Sweden, atlasantibodies.com) has developed the PrecisA Monoclonal Anti-PODXL antibodies — AMAb90643, AMAb90644, and AMAb90667. IHC has been used for extensive evaluation of these mouse monoclonal antibodies. The IHC staining pattern obtained from the urothelial and colorectal cancer tissues have been compared with the staining pattern of the corresponding polyclonal Anti-PODXL antibody (HPA002110) used in the colorectal studies discussed in this article. Consecutive sections of colorectal cancer tissue using the different Anti-PODXL antibodies are depicted in Figure 5.
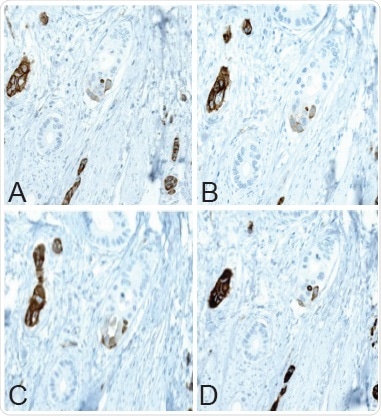
Figure 5. Immunohistochemical staining of PODXL protein in colorectal tumor tissue using (A) HPA002110, (B) AMAb90643, (C) AMAb90644, and (D) AMAb90667 antibodies.
Summary
- At present, there is a great need for innovative biomarkers with the potential to differentiate between different types, forms, and stages of colorectal as well as urothelial cancer.
- It has been found that PODXL is a prognostic biomarker in colorectal and urothelial cancer, where high membranous PODXL expression is an independent predictor of poor prognosis.
- Colorectal cancer patients can be stratified into those who must receive chemotherapy and who may be spared adjuvant treatment through the analysis of the PODXL expression.
References
- Uhlén M et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010 28(12):1248–50.
- Berglund L. et al. A gene-centric human protein atlas for expression profiles based on antibodies. Molecular & Cellular Proteomics. 2008 7:2019–2027.
- Asplund A. et al. Antibodies for profiling the human proteome - The Human Protein Atlas as a resource for cancer research. Proteomics. 2012 Jul;12(13):2067–77.
- Pontén F, Jirström K, Uhlén M. The Human Protein Atlas - a tool for pathology. J Pathology 2008 216(4):387–93.
- Larsson A. et al. Overexpression of podocalyxin-like protein is an independent factor of poor prognosis in colorectal cancer. Br J Cancer 2011 105(5):666–72.
- Boman K. et al. Membranous expression of podocalyxin-like protein is an independent factor of poor prognosis in urothelial bladder cancer. Br J Cancer 2013 108, 2321–2328.
To find the products listed in this article, please see the Atlas Antibodies product catalog.
Atlas Antibodies
Atlas Antibodies provides the antibodies from the Human Protein Atlas. We offer a wide range of highly validated research antibodies – Triple A Polyclonals and PrecisA Monoclonals – targeting over 75% of the human proteome.
Triple A Polyclonals are primary antibodies developed and characterized within the Human Protein Altas project. The 21,000 rabbit polyclonal antibodies cover 75% of the human proteome and are validated in IHC, WB and ICC-IF. For each IHC antibody, you can explore 500 IHC images for each antibody.
PrecisA Monoclonals are primary mouse monoclonals with unique antigen design for optimal specificity. They are epitope mapped with defined specificity and isotyped for multiplexing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last updated: Oct 29, 2018 at 10:29 AM


































No hay comentarios:
Publicar un comentario