
| Clinical Trials | ||
| Updates from the National Cancer Institute | ||
| Clinical Trials News | ||
 | For Small Cell Lung Cancer, Immunotherapy Drug Finally Brings Improved Survival In a large clinical trial, the immunotherapy drug atezolizumab (Tecentriq), combined with a standard chemotherapy regimen, increased survival in patients with advanced small cell lung cancer. The trial is the first in more than 20 years to show a survival improvement in this cancer. | |
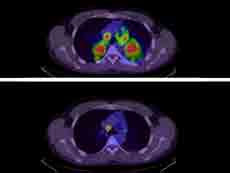 | Trial Results Highlight Changing Lung Cancer Treatment Landscape Results from two large clinical trials could cement the value of the drugs brigatinib (Alunbrig) and durvalumab (Imfinzi) in treating non-small cell lung cancer (NSCLC). The trial results, several experts said, confirm that the drugs can improve the outcomes of patients with advanced NSCLC. | |
 | Immunotherapy Drug Cemiplimab Approved for Advanced Squamous Cell Skin Cancer The Food and Drug Administration (FDA) has approved the immunotherapy drug cemiplimab (Libtayo) for an advanced form of cutaneous squamous cell carcinoma (SCC), a common type of skin cancer. FDA’s approval of cemiplimab was based on the results of two early-phase clinical trials. It is the first agent to be approved specifically for advanced SCC. | |
 | Find NCI-Supported Clinical Trials Use our search form to find a clinical trial or other research study that may be right for you or a loved one. | |
| Clinical Trials Information for Patients and Caregivers | ||
| How to Work With Your Health Insurance Plan This page highlights ways to learn if your health plan covers routine patient care costs in a clinical trial and offers ideas about who to contact for help, questions to ask, and information to collect and keep if you decide to take part in a trial. | ||
| Federal Government Programs Some federal programs help pay the costs of care in clinical trials. Beneficiaries of Medicare or TRICARE may have certain benefits related to clinical trial participation, and patients covered by the Department of Veterans Affairs may be eligible to participate in NCI-sponsored clinical trials. | ||
| NCI-Supported Clinical Trials That Are Recruiting Patients | ||
| Mulitargeted Fusion Protein for Relapsed Small Cell Lung Cancer This phase 1/2 trial is testing a fusion protein targeting two distinct molecular pathways combined with either topotecan or temozolomide for patients with relapsed small cell lung cancer. The fusion protein is an experimental immunotherapy drug that targets a pair of molecules that each dampen the immune system’s response to cancer. | ||
| Donor Stem Cell Transplantation for Primary Immunodeficiencies This pilot phase 2 trial will determine how well transplants of blood-forming stem cells from healthy donors work in people with primary immunodeficiency disorders. Doctors want to see if using a new approach to transplantation will overcome the risk of the patients' immune systems rejecting the transplant so that patients can develop healthy, new immune systems from the donor cells. | ||
| CAR T-Cell Therapy for Patients with Multiple Myeloma This phase 1 trial will determine the safety and best dose of CAR T cells, along with the chemotherapy drugs cyclophosphamide and fludarabine, for people with relapsed multiple myeloma, a cancer of blood plasma cells. The T cells will be genetically modified to target a protein called BCMA that is found on the surface of multiple myeloma cells. | ||


































No hay comentarios:
Publicar un comentario