
03/29/2016 09:00 AM EDT

When most of us come down with a bacterial infection, we generally bounce back with appropriate treatment in a matter of days. But that’s often not the case for people who suffer from common variable immunodeficiency (CVID), a group of rare disorders that increase the risk of life-threatening bacterial infections of the lungs, sinuses, and […]
Molecular Answers Found for a Mysterious Rare Immune Disorder
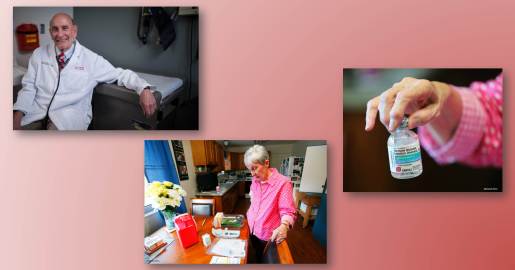
Caption: Helping to solve a medical mystery. Top left, University of Utah’s Harry Hill; Bottom, CVID patient Roma Jean Ockler; Right, Ockler showing the medication that helps to control her CVID.
Credit: Jeffrey Allred, Deseret News
Credit: Jeffrey Allred, Deseret News
When most of us come down with a bacterial infection, we generally bounce back with appropriate treatment in a matter of days. But that’s often not the case for people who suffer from common variable immunodeficiency (CVID), a group of rare disorders that increase the risk of life-threatening bacterial infections of the lungs, sinuses, and intestines. CVID symptoms typically arise in adulthood and often take many years to diagnose and treat, in part because its exact molecular causes are unknown in most individuals.
Now, by combining the latest in genomic technology with some good, old-fashioned medical detective work, NIH-funded researchers have pinpointed the genetic mutation responsible for an inherited subtype of CVID characterized by the loss of immune cells essential to the normal production of antibodies [1]. This discovery, reported recently in The New England Journal of Medicine, makes it possible at long last to provide a definitive diagnosis for people with this CVID subtype, paving the way for them to receive more precise medical treatment and care. More broadly, the new study demonstrates the power of precision medicine approaches to help the estimated 25 to 30 million Americans who live with rare diseases [2].
This research story began more than a decade ago. That’s when several physicians noticed CVID patients with very low numbers of B cells, the precursors of the body’s antibody-producing cells. All had recurrent infections and family members with similar problems, suggesting they might have the same subtype of CVID [3, 4].
The plot thickened a few years ago when Mary Ellen Conley, a researcher then at The Rockefeller University, New York, received blood and DNA samples from a mother and son with CVID-like systems and low B cell counts. Conley, known for her expertise in the genetics of B cell development, noted that no other family members showed any signs of CVID. She hypothesized that a new mutation had arisen in the mother and had then been passed to the son.
By sequencing the exomes—the 1.5 percent of the genome that codes for protein—of the mother, son, and his unaffected grandmother, Conley and her colleagues got their answer. The mother and son shared a defective copy of a gene called IKAROS, which encodes a transcription factor. Transcription factors bind DNA to influence the expression of other genes, and IKAROS was already known for its role in B cell development.
Through informal discussions of the discovery with other scientists, Conley soon came into contact with a research team at NIH’s Primary Immune Deficiency Clinic. The team included Sergio Rosenzweig, who, as hard work would have it, had identified another CVID family fitting the same description. Conley also learned that Harry Hill, a researcher at University of Utah Health Sciences, Salt Lake City and his colleagues had described a Utah woman with CVID named Roma Jean Ockler at a major immunology meeting in 2012. Ockler had three other family members with the condition, and Hill’s team had analyzed their genomes and discovered that all of the affected individuals carried a genomic deletion that spanned 11 genes. Among the missing genes: IKAROS.
These findings set in motion an international search for additional families and ultimately genetic analyses of blood samples at four laboratories in the United States. The effort turned up six families and a total of 29 individuals with mutations in IKAROS. Of the six familial mutations, all affected the ability of the gene’s transcription factor protein to bind DNA.
The researchers found that it only takes one faulty copy of IKAROS to produce the immune deficiency and susceptibility to infections seen in some people with this CVID subtype. Intriguingly, some people with the same mutations appear healthy. Geneticists call this phenomenon “incomplete penetrance.” The researchers hope that learning why that’s the case might help to understand the immune system better, perhaps pointing the way to new CVID treatments.
The findings now give this subtype its own stand-alone name as a rare immunologic disease: IKAROS deficiency. Already, the findings have improved genetic counseling for these families, but they aren’t expected to produce any immediate change in their medical treatment. Ockler and others with IKAROS deficiency will need to continue receiving frequent gamma globulin infusions to provide the antibodies their own systems are not making to provide temporary passive immunity to fight infections.
For a disease to be considered rare in the United States, it must affect no more than one in 200,000 people. And yet, when you add up all of the instances of the 7,000 or so recognized rare diseases together, you find that about 1 in 10 people has a rare condition of one kind or another [2]. In other words, collectively speaking, rare diseases aren’t so rare.
The good news for all those with rare, inherited diseases is the tools to find answers are getting better all the time. Ultimately, those insights will mean better, more precise medical care.
References:
[1] Loss of B Cells in Patients with Heterozygous Mutations in IKAROS. Kuehn HS, Boisson B, Cunningham-Rundles C, Reichenbach J, Stray-Pedersen A, Gelfand EW, Maffucci P, Pierce KR, Abbott JK, Voelkerding KV, South ST, Augustine NH, Bush JS, Dolen WK, Wray BB, Itan Y, Cobat A, Sorte HS, Ganesan S, Prader S, Martins TB, Lawrence MG, Orange JS, Calvo KR, Niemela JE, Casanova JL, Fleisher TA, Hill HR, Kumánovics A, Conley ME, Rosenzweig SD. N Engl J Med. 2016 Mar 17;374(11):1032-1043.
[2] Rare Diseases: Facts and Statistics. Global Genes.
[3] Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Dräger R, Eibel H, Fischer B, Schäffer AA, Mages HW, Kroczek RA, Peter HH. Nat Immunol. 2003 Mar;4(3):261-268.
[4] Deconstructing common variable immunodeficiency by genetic analysis. Schäffer AA, Salzer U, Hammarström L, Grimbacher B. Curr Opin Genet Dev. 2007 Jun;17(3):201-212.
Links:
Genetic and Rare Disease Information Center (National Center for Advancing Translational Sciences/NIH)
Common Variable Immunodeficiency (NCATS/NIH)
Primary Immune Deficiency Clinic (National Institute of Allergy and Infectious Diseases/NIH)
Harry Hill (University of Utah, Salt Lake City)
NIH Support: National Institute of Allergy and Infectious Diseases; National Institute of General Medical Sciences; National Human Genome Research Institute; Clinical Center





















.png)












No hay comentarios:
Publicar un comentario