

MedWatch Safety Information: Smart Lipo: Recall - Undeclared Drug Ingredients
SmartLipo365 is voluntarily recalling all lots of Smart Lipo (800, 900, 950 mg) capsules to the consumer level. FDA's analysis found the Smart Lipo products to contain undeclared sibutramine, desmethylsibutramine, and phenolphthalein. These undeclared ingredients make these products unapproved new drugs for which safety and efficacy have not been established. These products may also interact in life-threatening ways with other medications a consumer may be taking. More information
MedWatch Safety Information: Pink Bikini Dietary Supplement by Lucy's Weight Loss System: Recall - Undeclared Drug Ingredient
Lucy's Weight Loss System is voluntarily recalling all lots of Pink Bikini White powder Capsules, 30 white (750MG per capsule) to the consumer level. Pink Bikini has been found positive for diclofenac after FDA sampling and testing.
Diclofenac is a nonsteroidal anti-inflammatory drug (NSAID). This medicine works by reducing substances in the body that cause pain and inflammation. Diclofenac can increase your risk of fatal heart attack or stroke, especially if you use it long term or take high doses, or if you have heart disease. More information
|
Comunicaciones de la FDA sobre la seguridad de los medicamentos en español
Descargo de responsabilidad: La FDA reconoce la necesidad de proporcionar información importante sobre seguridad de los medicamentos en idiomas distintos al inglés. Hacemos lo mejor posible para proporcionar versiones en español precisas y oportunas de nuestras Comunicaciones de Seguridad de Medicamentos. Sin embargo, en caso que existiera discrepancias entre las versiones en inglés y la de español, la información contenida en la versión en inglés es la que se considera como versión oficial. Si tiene alguna pregunta, por favor contáctese con Division of Drug Information en druginfo@fda.hhs.gov.Comunicaciones de la FDA |


FDA recognizes the significant public health consequences that can result from drug shortages and takes tremendous efforts within its legal authority to address and prevent drug shortages. These shortages occur for many reasons, including manufacturing and quality problems, delays, and discontinuations. When issues are discovered by the company or the public and reported to FDA or are found by FDA upon inspection, FDA works closely with the firm to address risks involved to prevent harm to patients. FDA also considers the impact a shortage would have on patient care and access and works with the firm to restore supplies while also ensuring safety for patients. More information
|
Drug Shortages Voluntarily Reported by Manufacturers During the Past 2 Weeks:
- Eptifibatide (Integrilin) Injection
- TIgecycline (Tygacil) Injection
- LifeCare PCA™ Sterile Empty Vial and Injector
- Morphine Sulfate Injection, USP, CII, (Preservative-Free)(For PCA Use Only)
Drug Shortages Reported to be Resolved by Manufacturers During the Past 2 Weeks:
Drugs Reported to be Discontinued by Manufacturers During the Past 2 Weeks:
La FDA reconoce las consecuencias significativas para la salud pública que pueden resultar de la escasez de medicamentos y hace un gran esfuerzo dentro de sus facultades legales para abordar yprevenir la escasez de medicamentos. La escasez se produce por muchas razones, incluyendoproblemas de fabricación y calidad, retrasos y discontinuación del producto. Cuando los problemas son descubiertos por la empresa o el público y reportados a la FDA o se descubren por inspecciones de la FDA, la FDA trabaja en estrecha colaboración con la empresa para hacer frente a los riesgosinvolucrados y evitar daños a los pacientes. La FDA también considera el impacto que una escaseztendría en la atención médica del paciente y al acceso del producto y trabaja con la empresa pararestablecer el suministro al tiempo que garantiza la seguridad de los pacientes. Más información
|
Zurampic to treat high blood uric acid levels associated with gout
FDA approved Zurampic (lesinurad) to treat high levels of uric acid in the blood (hyperuricemia) associated with gout, when used in combination with a xanthine oxidase inhibitor (XOI), a type of drug approved to reduce the production of uric acid in the body.Gout is a painful form of arthritis caused by the buildup of too much uric acid in the body, and usually appears first as redness, soreness, and swelling in the big toe. More information |

New orphan drug approved to treat pulmonary arterial hypertension
FDA approved Uptravi (selexipag) tablets to treat adults with pulmonary arterial hypertension (PAH), a chronic, progressive, and debilitating rare lung disease that can lead to death or the need for transplantation.
“Uptravi offers an additional treatment option for patients with pulmonary arterial hypertension,” said Ellis Unger, M.D., director of the Office of Drug Evaluation I in the FDA’s Center for Drug Evaluation and Research. “The FDA supports continued efforts to provide new treatment options for rare diseases.”More information
|

FDA permits marketing of fecal continence restoration system
FDA approved the Fenix Continence Restoration System to treat fecal incontinence in patients who are not candidates for, or have previously failed, medical or other surgical options.
Fecal incontinence is the inability to control bowel movements. It is a common problem that is frequently underreported, especially among older adults. The most common cause of fecal incontinence is damage to the muscles around the anus (anal sphincter) from vaginal childbirth or functional disorders such as diabetes. More information
|

Wearable defibrillator for children at risk for sudden cardiac arrest approved
FDA approved a new indication for the LifeVest wearable cardioverter defibrillator. The LifeVest is approved for certain children who are at risk for sudden cardiac arrest, but are not candidates for an implantable defibrillator due to certain medical conditions or lack of parental consent.
While many automated external defibrillators (which require a second person to operate them) have been cleared for use in children, LifeVest is the only one worn by the patient and monitors the heart continuously for abnormal, life-threatening heart rhythms (arrhythmias). LifeVest responds automatically if it senses the need to deliver a shock, restoring a life-sustaining heartbeat. More information
|
Basaglar, approved as the first “follow-on” insulin glargine product to treat diabetes
FDA pproved Basaglar (insulin glargine injection), a long-acting human insulin analog to improve glycemic control in adult and pediatric patients with type 1 diabetes mellitus and in adults with type 2 diabetes mellitus.
According to the Centers for Disease Control and Prevention, approximately 21 million people in the United States have been diagnosed with diabetes. Over time, diabetes increases the risk of serious health complications, including heart disease, blindness, and nerve and kidney damage. Improvement in blood sugar control can reduce the risk of some of these long-term complications. More information
|
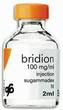
Bridion approved to reverse effects of neuromuscular blocking drugs used during surgery
FDA approved Bridion (sugammadex) injection to reverse the effects of neuromuscular blockade induced by rocuronium bromide and vecuronium bromide, which are used during certain types of surgery in adults. Rocuronium bromide and vecuronium bromide are neuromuscular blocking drugs that cause temporary paralysis by interfering with the transmission of nerve impulses to the muscle and are used to paralyze the vocal cords when patients require an artificial airway or breathing tube for surgery, a process called tracheal intubation. More information |
First emergency treatment for overdose of certain types of chemotherapy approved
FDA approved Vistogard (uridine triacetate) for the emergency treatment of adults and children who receive an overdose of the cancer treatment fluorouracil or capecitabine, or who develop certain severe or life-threatening toxicities within four days of receiving these cancer treatments.“Treating cancer requires not only selecting which drug may be most effective and well tolerated, but ensuring the correct dose is given at proper intervals. While rare, unintentional overdose can occur,” said Richard Pazdur, M.D., director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. More information |

New oral therapy to treat ALK-positive lung cancer approved
FDA approved Alecensa (alectinib) to treat people with advanced (metastatic) ALK-positive non-small cell lung cancer (NSCLC) whose disease has worsened after, or who could not tolerate treatment with, another therapy called Xalkori (crizotinib).Lung cancer is the leading cause of cancer death in the United States, with an estimated 221,200 new diagnoses and 158,040 deaths in 2015, according to the National Cancer Institute. An ALK (anaplastic lymphoma kinase) gene mutation can occur in several different types of cancer cells, including lung cancer cells. More information |
For information on drug approvals or to view prescribing information and patient information, please visit Drugs@FDA or DailyMed.

View FDA's Comments on Current Draft Guidance page, for a list of current draft guidances and other topics of interest for patients and caregivers.
|

FDA Launches precisionFDA to Harness the Power of Scientific Collaboration, by: Taha A. Kass-Hout, M.D., M.S., is FDA’s Chief Health Informatics Officer and Director of FDA’s Office of Health Informatics. Elaine Johanson is the precisionFDA Project Manager.
Imagine a world where doctors have at their fingertips the information that allows them to individualize a diagnosis, treatment or even a cure for a person based on their genes. That’s what President Obama envisioned when he announced his Precision Medicine Initiative earlier this year. Today, with the launch of FDA’s precisionFDA web platform, we’re a step closer to achieving that vision.
precisionFDA is an online, cloud-based, portal that will allow scientists from industry, academia, government and other partners to come together to foster innovation and develop the science behind a method of “reading” DNA known as next-generation sequencing (or NGS). Next Generation Sequencing allows scientists to compile a vast amount of data on a person’s exact order or sequence of DNA.
To continue reading this blog, see FDAVoice Blog posted on December 15, 2015
|

FDA updates blood donor deferral policy to reflect the most current scientific evidence and continue to ensure the safety of the U.S. blood supply
The FDA issued final guidance outlining updated blood donor deferral recommendations to reflect the most current scientific evidence and to help ensure continued safety of the blood supply by reducing the risk of human immunodeficiency virus (HIV) transmission by blood and blood products.
“The FDA’s responsibility is to maintain a high level of blood product safety for people whose lives depend on it,” said the FDA’s Acting Commissioner Stephen Ostroff, M.D. “We have taken great care to ensure this policy revision is backed by sound science and continues to protect our blood supply.” More information
|

FDA proposes tanning bed age restrictions and other important safety measures
FDA announced important proposed steps to protect public health by preventing the use of sunlamp products (also commonly known as indoor tanning beds) by minors and reducing the risk of using these devices for adults. The FDA is committed to protecting public health by informing consumers of the risks of indoor tanning.
“Today’s action is intended to help protect young people from a known and preventable cause of skin cancer and other harms,” said acting FDA Commissioner Stephen Ostroff, M.D. “Individuals under 18 years are at greatest risk of the adverse health consequences of indoor tanning.” More information and to read the FDA Consumer Update Article
|

FDA advisory committee meetings are free and open to the public. No prior registration is required to attend. Interested persons may present data, information, or views, orally at the meeting, or in writing, on issues pending before the committee.
Other types of meetings listed may require prior registration and fees.
View FDA's Calendar of Public Meetings page for a complete list of meetings and workshops.
|
Public Workshop: Point of Care Prothrombin Time/International Normalized Ratio Devices for Monitoring Warfarin Therapy
Date: January 25, 2016, 8:00 am to 5:00 pm
Agenda:The purpose of this workshop is to discuss and receive input from stakeholders regarding approaches to the analytical and clinical validation of point of care (POC) Prothrombin Time/International Normalized Ratio (PT/INR) in vitro diagnostic devices for improved clinical management of warfarin therapy in addition to describing the FDA’s process for facilitating the development of safe and effective POC and patient self-testing PT/INR devices.
Date: January 25, 2016, 8:00 am to 5:00 pm
Agenda:The purpose of this workshop is to discuss and receive input from stakeholders regarding approaches to the analytical and clinical validation of point of care (POC) Prothrombin Time/International Normalized Ratio (PT/INR) in vitro diagnostic devices for improved clinical management of warfarin therapy in addition to describing the FDA’s process for facilitating the development of safe and effective POC and patient self-testing PT/INR devices.
Please visit FDA’s Advisory Committee page to obtain advisory committee meeting agendas, briefing materials, and meeting rosters prior to the meetings. You may also visit this page after meetings to obtain transcripts, presentations, and voting results. For additional information on other agency meetings please visit Meetings, Conferences, & Workshops.
|

Keep Your Dogs and Cats Safe From Holiday Hazards
This holiday season, while you’re busy decorating, cooking, and wrapping gifts, remember to watch out for holiday temptations for your pets. FDA veterinarian Carmela Stamper tells how to keep your animals safe.
If your dog received a stocking full of pet treats, make sure he doesn’t gobble them all up at once. According to Stamper, if he eats the treats whole, or eats too many at once, he may not be able to digest them. Unchewed pet treats can get stuck in the trachea (windpipe) or gastrointestinal tract (esophagus, stomach, and intestines), particularly in small dogs. More information
|
More Consumer Updates
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español

FDA takes action against Vermont dairy farm for illegally administering drugs to cattle
The U.S. Department of Justice entered a consent decree of permanent injunction in the District of Vermont against the Correia Family Limited Partnership, doing business as Wynsum Holsteins, a dairy farm located in West Addison, Vermont, and its partners, Anthony Correia, Barbara Correia and Stephen Correia. The decree accompanies a complaint filed at the request of the U.S. Food and Drug Administration.
An FDA inspection conducted between November and December 2014 revealed that Wynsum Holsteins violated several provisions of the Federal Food, Drug, and Cosmetic Act (FD&C Act). Among those violations, the FDA found that the company offered for sale cows with illegal drug residues in their tissue, administered drugs contrary to label directions without proper veterinary oversight and supervision and failed to maintain adequate treatment records. More information
|

Center for Food Safety and Applied Nutrition
The Center for Food Safety and Applied Nutrition, known as CFSAN, carries out the mission of FDA. The Center provides services to consumers, domestic and foreign industry and other outside groups regarding field programs; agency administrative tasks; scientific analysis and support; and policy, planning and handling of critical issues related to food and cosmetics. More information
Food Facts for You
The Center for Food Safety and Applied Nutrition, known as CFSAN, issues food facts for consumers to keep you and your family safe. More information |

Animal Health Literacy
Animal Health Literacy means timely information for the benefit of all animals and their humans. With continuous communication and outreach, the Center for Veterinary Medicine (CVM) strives to enhance the public trust, promote safe and effective use of the animal health products we regulate, and share our scientific endeavors. CVM provides reliable, science-based information to promote animal and human health. More information and Publicaciones en Español del
Animal and Veterinary Updates
Animal and veterinary updates provide information to keep your pets healthy and safe. More information |

How to Report a Pet Food Complaint
You can report complaints about a pet food product electronically through the Safety Reporting Portal or you can call your state’s FDA Consumer Complaint Coordinators. Please provide as much information as possible in your complaint, such as exact name of product, type of container, lot number, UPC codes, how the food was stored, and purchase date and exact location where purchased. If possible, please save the original packaging until the pet food has been consumed. The packaging contains IMPORTANT information often needed to identify the variety of pet food, the manufacturing plant, and the production date.More information |

Public Health Education
Tobacco products are harmful, yet widely used, consumer products that are responsible for severe health problems in both users and non-users. These health problems include cancer, lung disease, and heart disease, which often lead to death.
|
Public Education CampaignsWe are investing in a number of public education campaigns, such as Fresh Empire and The Real Cost, to help educate the public – especially youth – about the dangers of regulated tobacco products. Rooted in science, these efforts are directly linked to our authority to regulate the marketing and sales of tobacco products. More information
Youth and TobaccoWe are working to protect the health of America’s children and ultimately reduce the burden of illness and death caused by tobacco use. More information

Information about Expanded Access
Expanded access, sometimes called "compassionate use," is the use outside of a clinical trial of an investigational medical product (i.e., one that has not been approved by FDA). FDA is committed to increasing awareness of and knowledge about its expanded access programs and the procedures for obtaining access to human investigational drugs (including biologics) and medical devices. More information |
Learn about what your physician should do before submitting a request for individual patient expanded access use of an investigational medical product, who may be eligible for expanded access, associated costs, FDA contacts and more. Information for Patients
|
Learn about your responsibilities under the expanded access pathway, how to submit a request for expanded access for an individual patient (including for emergency use), which forms to use, FDA contacts and more. Information for Physicians
|

Patient Network Webinars
Through our webinars and presentations, the Office of Health and Constituent Affairs brings information to you on many topics related to patient engagement, medical product (Drugs, Biologics, Devices) approval and medical product safety updates. More information
FDA Basics
Each month, different centers and offices at FDA will host an online session where the public can ask questions to senior FDA officials about a specific topic or just listen in to learn more about FDA. More information
Educational Videos
|

healthfinder.gov
Welcome to healthfinder.gov, a government Web site where you will find information and tools to help you and those you care about stay healthy.More information /más información
FDA E-list
Sign up for one of the FDA disease specific e-mail list that delivers updates, including product approvals, safety warnings, notices of upcoming meetings, and notices on proposed regulatory guidances.
You may wish to sign up for other email updates from the FDA - see other email updates.
|
Patient Network - Bring Your Voice to FDA
An interactive tool for educating patients, patient advocates, and consumers on how their medications - both prescription and over-the-counter - and medical devices move from the realm of idea to the realm of the marketplace. More information |


































No hay comentarios:
Publicar un comentario