
 Drug Safety Communication: SGLT2 Inhibitors for diabetes - Labels to Include Warnings About Too Much Acid in the Blood and Serious Urinary Tract InfectionsAn FDA safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose cotransporter-2 (SGLT2) inhibitors about the risks of too much acid in the blood and of serious urinary tract infections. Both conditions can result in hospitalization. FDA issued a Drug Safety Communication in May 2015 warning about the risk of ketoacidosis with SGLT2 inhibitors and alerting that the Agency would continue to evaluate this safety issue. More information
OmniPod Insulin Management System by Insulet: Field Safety Notification - Reported Cases of Needle Mechanism Deployment Failure or DelayInsulet Corporation initiated a lot-specific voluntary Field Safety Notification (Notification) for 15 lots of the OmniPod (Pod) which were distributed in the U.S. and three lots which were distributed internationally. This Notification is due to a slight increase in the reported cases in which the Pod’s needle mechanism failed to deploy or there was a delay in the deployment of the needle mechanism. In the event a needle mechanism fails to deploy, the needle will not be inserted and insulin delivery will not begin. The interruption of insulin delivery may cause elevated blood glucose (hyperglycemia), which, if left untreated, can result in diabetic ketoacidosis (DKA). See the Press Release for a listing of affected product lots.
More information
Recall: Bestmed, LLC Digital Temple Thermometer (DTT™), Model No. KD-2201 Manufactured By K-Jump Health Co., Ltd.Bestmed, LLC, a medical device distributor, initiated a nationwide recall of the Digital Temple Thermometer Model No. KD-2201 manufactured by K-Jump Health Co., Ltd, featuring lot numbers S/N: 3612 through S/N: 3715, which were sold between October 2012 until the start of the recall in November 2015. Some Digital Temple Thermometers contain a manufacturing problem causing the affected thermometers to display temperatures that are inaccurate and lower than actual body temperatures, which potentially may cause the user or caregiver of the user to delay or forego seeking appropriate care (generally an over-the-counter fever reduction medication) or receive more care than appropriate, when relying solely on the temperature display on the thermometer.
More information |

Recall: Lipo Escultura - Undeclared Drug Ingredients
Lipo Escultura Corp. of Brooklyn, NY (dba JAT Productos Naturales Corp., and JAT Natural Products Corp.) are voluntarily recalling all Lipo Escultura within expiry to the consumer level. The Lipo Escultura capsules were tested by the FDA and have been found to contain sibutramine and diclofenac. Sibutramine is an appetite suppressant now a controlled substance that was removed from the market for safety reasons. Sibutramine is known to substantially increase blood pressure and/or pulse rate in some patients and may present a significant risk for patients with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke. Diclofenac is a non-steroidal anti-inflammatory drug (commonly referred to as NSAIDs). NSAIDS may cause increased risk of cardiovascular events, such as heart attack and stroked, as well as serious gastrointestinal damage, including bleeding, ulceration, and fatal perforation of the stomach and intestines. More information
Recall: Compounded Multivitamins by Glades Drugs - High Amounts of Vitamin D3 (Cholecalciferol)
FDA is alerting health care professionals and patients of a voluntary recall of compounded multivitamin capsules containing high amounts of Vitamin D3 (Cholecalciferol), distributed nationwide by Glades Drugs in Pahokee, Florida. FDA has received reports of several adverse events potentially associated with these compounded capsules made by Glades Drugs. Consumption of this product may result in vitamin D toxicity, which may be severe and may lead to life-threatening outcomes if left untreated. Patients who have received these compounded capsules should stop taking this medication and immediately seek medical attention. More information |
Comunicaciones de la FDA sobre la seguridad de los medicamentos en español
Descargo de responsabilidad: La FDA reconoce la necesidad de proporcionar información importante sobre seguridad de los medicamentos en idiomas distintos al inglés. Hacemos lo mejor posible para proporcionar versiones en español precisas y oportunas de nuestras Comunicaciones de Seguridad de Medicamentos. Sin embargo, en caso que existiera discrepancias entre las versiones en inglés y la de español, la información contenida en la versión en inglés es la que se considera como versión oficial. Si tiene alguna pregunta, por favor contáctese con Division of Drug Information en druginfo@fda.hhs.gov. Comunicaciones de la FDA |


FDA recognizes the significant public health consequences that can result from drug shortages and takes tremendous efforts within its legal authority to address and prevent drug shortages. These shortages occur for many reasons, including manufacturing and quality problems, delays, and discontinuations. When issues are discovered by the company or the public and reported to FDA or are found by FDA upon inspection, FDA works closely with the firm to address risks involved to prevent harm to patients. FDA also considers the impact a shortage would have on patient care and access and works with the firm to restore supplies while also ensuring safety for patients.
More information |
Drugs Reported to be Discontinued by Manufacturers During the Past 2 Weeks:
La FDA reconoce las consecuencias significativas para la salud pública que pueden resultar de la escasez de medicamentos y hace un gran esfuerzo dentro de sus facultades legales para abordar yprevenir la escasez de medicamentos. La escasez se produce por muchas razones, incluyendoproblemas de fabricación y calidad, retrasos y discontinuación del producto. Cuando los problemas son descubiertos por la empresa o el público y reportados a la FDA o se descubren por inspecciones de la FDA, la FDA trabaja en estrecha colaboración con la empresa para hacer frente a los riesgosinvolucrados y evitar daños a los pacientes. La FDA también considera el impacto que una escaseztendría en la atención médica del paciente y al acceso del producto y trabaja con la empresa pararestablecer el suministro al tiempo que garantiza la seguridad de los pacientes. Más información
|

FDA approves Empliciti, a new immune-stimulating therapy to treat multiple myeloma
FDA has granted approval for Empliciti (elotuzumab) in combination with two other therapies to treat people with multiple myeloma who have received one to three prior medications. Multiple myeloma is a form of blood cancer that occurs in infection-fighting plasma cells (a type of white blood cell) found in the bone marrow. These cancerous cells multiply, produce an abnormal protein and push out other healthy blood cells from the bone marrow. This disease may result in a weakened immune system, and cause other bone and kidney problems. More information
FDA approves first drug to treat a rare enzyme disorder in pediatric and adult patients
FDA has approved Kanuma (sebelipase alfa) as the first treatment for patients with a rare disease known as lysosomal acid lipase (LAL) deficiency. Patients with LAL deficiency (also known as Wolman disease and cholesteryl ester storage disease [CESD]) have no or little LAL enzyme activity. This results in a build-up of fats within the cells of various tissues that can lead to liver and cardiovascular disease and other complications. More information
FDA approves first recombinant von Willebrand factor to treat bleeding episodes
FDA has approved Vonvendi, von Willebrand factor (Recombinant), for use in adults 18 years of age and older who have von Willebrand disease (VWD). Vonvendi is the first FDA-approved recombinant von Willebrand factor, and is approved for the on-demand (as needed) treatment and control of bleeding episodes in adults diagnosed with VWD. VWD is the most common inherited bleeding disorder, affecting approximately 1 percent of the U.S. population. More information |

FDA allows marketing of cooling cap to reduce hair loss during chemotherapy
FDA has cleared for marketing in the United States the first cooling cap to reduce hair loss (alopecia) in female breast cancer patients undergoing chemotherapy. Hair loss is a common side effect of certain types of chemotherapy, commonly associated with the treatment of breast cancer. Hair may fall out entirely, gradually, in sections, or may become thin. Hair loss due to cancer treatment is usually temporary, but minimizing or relieving these kinds of side effects are considered important to overall treatment. More information
FDA clears military traumatic wound dressing for use in the civilian populationFDA has cleared the use of the XSTAT 30 wound dressing, an expandable, multi-sponge dressing used to control severe, life-threatening bleeding from wounds in areas that a tourniquet cannot be placed (such as the groin or armpit) in battlefield and civilian trauma settings. The clearance expands the device’s indication from use by the military only to use in adults and adolescents in the general population.
More information |
For information on drug approvals or to view prescribing information and patient information, please visit Drugs@FDA or DailyMed.

View FDA's Comments on Current Draft Guidance page, for a list of current draft guidances and other topics of interest for patients and caregivers.
|

Lessons Learned in Mexico about Food Safety – And Tomatoes, by Stephen M. Ostroff, M.D., Acting Commissioner of Food and Drugs
As my colleagues at FDA can attest, I like to grow tomatoes in the summer. I often bring portions of the harvest to the office each week of the growing season. Though the total volume is modest, I still like to think of myself as an environmentally conscious and responsible “farmer.” So I was glad to spend some time on food safety issues last month while in Mexico attending the 10th International Summit of Heads of Medicines Regulatory Agencies. In addition to summit activities, I spent time with FDA staff in our Latin America Office and with our regulatory counterparts in Mexico charged with keeping foods safe. In the process, I gained a new appreciation for the partnership we have with Mexico to enhance food safety and to minimize the potential for contamination of fresh produce.
To read the rest of this post,
see FDA Voice Blog, December 1, 2015. |

FDA advisory committee meetings are free and open to the public. No prior registration is required to attend. Interested persons may present data, information, or views, orally at the meeting, or in writing, on issues pending before the committee.
Other types of meetings listed may require prior registration and fees.
View FDA's Calendar of Public Meetings page for a complete list of meetings and workshops.
|
Please visit FDA’s Advisory Committee page to obtain advisory committee meeting agendas, briefing materials, and meeting rosters prior to the meetings. You may also visit this page after meetings to obtain transcripts, presentations, and voting results. For additional information on other agency meetings please visit Meetings, Conferences, & Workshops.
|
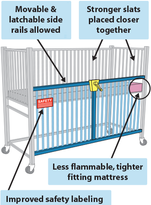
Help Keep a Sick Child Safe: Learn How to Use a Hospital Crib
As any parent whose baby has spent some time in the hospital knows, all cribs are not created equal. In most cases, hospital cribs (also called pediatric medical cribs) differ significantly from what’s in your child’s bedroom at home. “Each type of crib is specially designed for safe use in the environment in which it is being used,” says Victoria Wagman, M.A., a senior science health advisor at the Food and Drug Administration (FDA). And it’s important that you, as a parent, know how to use these cribs correctly, both in and out of the hospital—especially if your special needs child uses one of these cribs at home. More information |
More Consumer Updates
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español

FDA Has Determined That the AquAdvantage Salmon is as Safe to Eat as Non-GE Salmon
After an exhaustive and rigorous scientific review, FDA has arrived at the decision that AquAdvantage salmon is as safe to eat as any non-genetically engineered (GE) Atlantic salmon, and also as nutritious. The FDA scientists rigorously evaluated extensive data submitted by the manufacturer, AquaBounty Technologies, and other peer-reviewed data, to assess whether AquAdvantage salmon met the criteria for approval established by law; namely, safety and effectiveness. The data demonstrated that the inserted genes remained stable over several generations of fish, that food from the GE salmon is safe to eat by humans and animals, that the genetic engineering is safe for the fish, and the salmon meets the sponsor’s claim about faster growth. More information
Center for Food Safety and Applied NutritionThe Center for Food Safety and Applied Nutrition, known as CFSAN, carries out the mission of FDA. The Center provides services to consumers, domestic and foreign industry and other outside groups regarding field programs; agency administrative tasks; scientific analysis and support; and policy, planning and handling of critical issues related to food and cosmetics. More information
Food Facts for YouThe Center for Food Safety and Applied Nutrition, known as CFSAN, issues food facts for consumers to keep you and your family safe.
More information |

No Bones About It: Reasons Not to Give Your Dog Bones
You’ve just finished a big weekend family dinner and you are wondering what to do with the bones from the ham and roast, when in trots your big black Labrador Retriever. It’s hard to resist those longing, puppy-dog eyes. Your veterinarian has told you it’s a bad idea to give bones to your dog, but you’ve done so in the past with no harm done. What to do? More information
Animal Health LiteracyAnimal Health Literacy means timely information for the benefit of all animals and their humans. With continuous communication and outreach, the Center for Veterinary Medicine (CVM) strives to enhance the public trust, promote safe and effective use of the animal health products we regulate, and share our scientific endeavors. CVM provides reliable, science-based information to promote animal and human health.
More information and Publicaciones en Español del
Animal and Veterinary UpdatesAnimal and veterinary updates provide information to keep your pets healthy and safe. More information
|

How to Report a Pet Food Complaint
You can report complaints about a pet food product electronically through the Safety Reporting Portal or you can call your state’s FDA Consumer Complaint Coordinators.Please provide as much information as possible in your complaint, such as exact name of product, type of container, lot number, UPC codes, how the food was stored, and purchase date and exact location where purchased. If possible, please save the original packaging until the pet food has been consumed. The packaging contains IMPORTANT information often needed to identify the variety of pet food, the manufacturing plant, and the production date. More information |

Public Health Education
Tobacco products are harmful, yet widely used, consumer products that are responsible for severe health problems in both users and non-users. These health problems include cancer, lung disease, and heart disease, which often lead to death.
|
Public Education Campaigns
We are investing in a number of public education campaigns, such as Fresh Empire and The Real Cost, to help educate the public – especially youth – about the dangers of regulated tobacco products. Rooted in science, these efforts are directly linked to our authority to regulate the marketing and sales of tobacco products. More information
We are investing in a number of public education campaigns, such as Fresh Empire and The Real Cost, to help educate the public – especially youth – about the dangers of regulated tobacco products. Rooted in science, these efforts are directly linked to our authority to regulate the marketing and sales of tobacco products. More information
Youth and Tobacco
We are working to protect the health of America’s children and ultimately reduce the burden of illness and death caused by tobacco use. More information
We are working to protect the health of America’s children and ultimately reduce the burden of illness and death caused by tobacco use. More information

Information about Expanded Access
Expanded access, sometimes called "compassionate use," is the use outside of a clinical trial of an investigational medical product (i.e., one that has not been approved by FDA). FDA is committed to increasing awareness of and knowledge about its expanded access programs and the procedures for obtaining access to human investigational drugs (including biologics) and medical devices. More information |
Learn about what your physician should do before submitting a request for individual patient expanded access use of an investigational medical product, who may be eligible for expanded access, associated costs, FDA contacts and more. Information for Patients
|
Learn about your responsibilities under the expanded access pathway, how to submit a request for expanded access for an individual patient (including for emergency use), which forms to use, FDA contacts and more. Information for Physicians
|

Patient Network Webinars
Through our webinars and presentations, the Office of Health and Constituent Affairs brings information to you on many topics related to patient engagement, medical product (Drugs, Biologics, Devices) approval and medical product safety updates. More information
FDA Basics
Each month, different centers and offices at FDA will host an online session where the public can ask questions to senior FDA officials about a specific topic or just listen in to learn more about FDA. More information
Educational Videos
|

healthfinder.gov
Welcome to healthfinder.gov, a government Web site where you will find information and tools to help you and those you care about stay healthy.More information /más información
FDA E-list
Sign up for one of the FDA disease specific e-mail list that delivers updates, including product approvals, safety warnings, notices of upcoming meetings, and notices on proposed regulatory guidances.
You may wish to sign up for other email updates from the FDA - see other email updates.
|
Patient Network - Bring Your Voice to FDA
An interactive tool for educating patients, patient advocates, and consumers on how their medications - both prescription and over-the-counter - and medical devices move from the realm of idea to the realm of the marketplace. More information |





















.png)












No hay comentarios:
Publicar un comentario