

Drug Safety Communication: Gilenya (fingolimod) - FDA Warns About Cases of Rare Brain Infection
FDA is warning that a case of definite progressive multifocal leukoencephalopathy (PML) and a case of probable PML have been reported in patients taking Gilenya (fingolimod) for multiple sclerosis (MS). These are the first cases of PML reported in patients taking Gilenya who had not been previously treated with an immunosuppressant drug for MS or any other medical condition. As a result, information about these recent cases is being added to the drug label.More information |

MedWatch Alert: Hydrochlorothiazide Tablets by Unichem Pharmaceuticals (USA), Inc: Recall - Potential Presence of Foreign Tablets Contamination
Unichem Pharmaceuticals (USA), Inc. (Unichem) is voluntarily recalling one lot of Hydrochlorothiazide Tablets 25 mg 1000-count bottle to the consumer level. This recall has been initiated as a precautionary measure due to the identification of a Clopidogrel tablet found in a bottle of the product. The risk associated with mistakenly taking a Clopidogrel tablet instead of a Hydrochlorothiazide tablet is the increased probability of experiencing Clopidogrel's side effects which include bleeding and/or bruising. Patients with active bleeding or who are allergic to Clopidogrel or any component of the formulation may experience more serious adverse health consequences as a result of unknowingly consuming Clopidogrel. Additionally, missing a dose of Hydrochlorothiazide could result in uncontrolled blood pressure or swelling caused by excess fluid (edema). More information
MedWatch Alert: Symbiq Infusion System by Hospira - Cybersecurity Vulnerabilities
The FDA, the U.S. Department of Homeland Security’s Industrial Control Systems Cyber Emergency Response Team (ICS-CERT), and Hospira are aware of cybersecurity vulnerabilities associated with the Symbiq Infusion System. FDA strongly encourages health care facilities transition to alternative infusion systems, and discontinue use of these pumps.
Hospira and an independent researcher confirmed that Hospira’s Symbiq Infusion System could be accessed remotely through a hospital’s network. This could allow an unauthorized user to control the device and change the dosage the pump delivers, which could lead to over- or under-infusion of critical patient therapies. The FDA and Hospira are currently not aware of any patient adverse events or unauthorized access of a Symbiq Infusion System in a health care setting. More information
|

MedWatch Alert: Brintellix (vortioxetine) and Brilinta (ticagrelor): Drug Safety Communication - Name Confusion
FDA is warning health care professionals and patients that reports of confusion between the antidepressant Brintellix and anti-blood clotting medication Brilinta have resulted in the wrong medication being prescribed or dispensed. FDA determined that the main reason for the confusion between these two medications is the similarity of their brand (proprietary) names. None of the reports indicates that a patient ingested the wrong medication; however, reports of prescribing and dispensing errors continue. Health care professionals can reduce the risk of name confusion by including the generic (established) name of the medication, in addition to the brand name, and the indication for use when prescribing these medications. Patients should check their prescriptions to ensure that the correct medication was dispensed. More information |
Comunicaciones de la FDA sobre la seguridad de los medicamentos en español
Descargo de responsabilidad: La FDA reconoce la necesidad de proporcionar información importante sobre seguridad de los medicamentos en idiomas distintos al inglés. Hacemos lo mejor posible para proporcionar versiones en español precisas y oportunas de nuestras Comunicaciones de Seguridad de Medicamentos. Sin embargo, en caso que existiera discrepancias entre las versiones en inglés y la de español, la información contenida en la versión en inglés es la que se considera como versión oficial. Si tiene alguna pregunta, por favor contáctese con Division of Drug Information endruginfo@fda.hhs.gov. Comunicaciones de la FDA |

FDA recognizes the significant public health consequences that can result from drug shortages and takes tremendous efforts within its legal authority to address and prevent drug shortages. These shortages occur for many reasons, including manufacturing and quality problems, delays, and discontinuations. When issues are discovered by the company or the public and reported to FDA or are found by FDA upon inspection, FDA works closely with the firm to address risks involved to prevent harm to patients. FDA also considers the impact a shortage would have on patient care and access and works with the firm to restore supplies while also ensuring safety for patients. More information
|
Drug Shortages Voluntarily Reported by Manufacturers During the Past 2 Weeks:
Drug Shortages Reported to be Resolved by Manufacturers During the Past 2 Weeks:
Drugs Reported to be Discontinued by Manufacturers During the Past 2 Weeks:
La FDA reconoce las consecuencias significativas para la salud pública que pueden resultar de la escasez de medicamentos y hace un gran esfuerzo dentro de sus facultades legales para abordar yprevenir la escasez de medicamentos. La escasez se produce por muchas razones, incluyendoproblemas de fabricación y calidad, retrasos y discontinuación del producto. Cuando los problemas son descubiertos por la empresa o el público y reportados a la FDA o se descubren por inspecciones de la FDA, la FDA trabaja en estrecha colaboración con la empresa para hacer frente a los riesgosinvolucrados y evitar daños a los pacientes. La FDA también considera el impacto que una escaseztendría en la atención médica del paciente y al acceso del producto y trabaja con la empresa pararestablecer el suministro al tiempo que garantiza la seguridad de los pacientes. Más información
|

Non-surgical temporary balloon device approved to treat obesity
The FDA approved a new balloon device to treat obesity without the need for invasive surgery. The ReShape Integrated Dual Balloon System (ReShape Dual Balloon) is intended to facilitate weight loss in obese adult patients. The device likely works by occupying space in the stomach, which may trigger feelings of fullness, or by other mechanisms that are not yet understood.
The ReShape Dual Balloon device is delivered into the stomach via the mouth through a minimally invasive endoscopic procedure. The outpatient procedure usually takes less than 30 minutes while a patient is under mild sedation. Once in place, the balloon device is inflated with a sterile solution, which takes up room in the stomach. More information
|

Praluent approved to treat certain patients with high cholesterol
FDA approved Praluent (alirocumab) injection, the first cholesterol-lowering treatment approved in a new class of drugs known as proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitors.
Praluent is approved for use in addition to diet and maximally tolerated statin therapy in adult patients with heterozygous familial hypercholesterolemia (HeFH) or patients with clinical atherosclerotic cardiovascular disease such as heart attacks or strokes, who require additional lowering of LDL cholesterol. More information
|

New treatment for most common form of advanced skin cancer approved
The FDA approved Odomzo (sonidegib) to treat patients with locally advanced basal cell carcinoma that has recurred following surgery or radiation therapy, or who are not candidates for surgery or radiation therapy. Skin cancer is the most common cancer and basal cell carcinoma accounts for approximately 80 percent of non-melanoma skin cancers. Basal cell carcinoma starts in the top layer of the skin (called the epidermis) and usually develops in areas that have been regularly exposed to the sun and other forms of ultraviolet radiation. More information |

New treatment for chronic hepatitis C genotype 3 infections
FDA approved Daklinza (daclatasvir) for use with sofosbuvir to treat hepatitis C virus (HCV) genotype 3 infections. Daklinza is the first drug that has demonstrated safety and efficacy to treat genotype 3 HCV infections without the need for co-administration of interferon or ribavirin, two FDA-approved drugs also used to treat HCV infection. Hepatitis C is a viral disease that causes inflammation of the liver that can lead to diminished liver function or liver failure. Most people infected with HCV have no symptoms of the disease until liver damage becomes apparent, which may take several years. More information |

Technivie approved for treatment of chronic hepatitis C genotype 4
The FDA approved Technivie (ombitasvir, paritaprevir and ritonavir) for use in combination with ribavirin for the treatment of hepatitis C virus (HCV) genotype 4 infections in patients without scarring and poor liver function (cirrhosis).
Technivie in combination with ribavirin is the first drug that has demonstrated safety and efficacy to treat genotype 4 HCV infections without the need for co-administration of interferon, an FDA-approved drug also used to treat HCV infection. More information
Hepatitis B and C email updates
Sign up for the FDA Hepatitis B and C e-mail list that delivers updates, including product approvals, safety warnings, notices of upcoming meetings, and notices on proposed regulatory guidances. |

Diagnostic test approved to differentiate between types of HIV infection
The FDA approved the Bio-Rad BioPlex 2200 HIV Ag-Ab assay, the first FDA-approved diagnostic that differentiates between HIV-1 antibodies, HIV-2 antibodies, and HIV-1 p24 antigen in human serum or plasma specimens.
Two major types of HIV have been identified: HIV-1 and HIV-2. HIV-1 is responsible for most HIV infections throughout the world. HIV-2 is found primarily in West Africa; however, cases of HIV-2 infection have been identified in the United States. HIV-1 and HIV-2 are similar, but distinct viruses. More information
|
For information on drug approvals or to view prescribing information and patient information, please visit Drugs@FDA or DailyMed.

View FDA's Comments on Current Draft Guidance page, for a list of current draft guidances and other topics of interest for patients and caregivers.
|
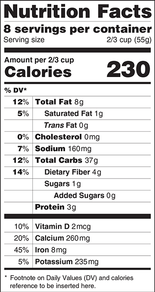
FDA revises proposed Nutrition Facts label rule to include a daily value for added sugars
The FDA proposed including the percent daily value (%DV) for added sugars on the Nutrition Facts label of packaged foods, giving consumers additional information for added sugars similar to information they have seen for decades with respect to nutrients such as sodium and certain fats. The percent daily value indicates how much a nutrient in a serving of food contributes to a daily diet and would help consumers make informed choices for themselves and their families. The percent daily value would be based on the recommendation that the daily intake of calories from added sugars not exceed 10 percent of total calories.
The proposed rule is a supplement to the March 3, 2014 proposed rule on updating the Nutrition Facts label, under which the FDA proposed that food companies include added sugars on the Nutrition Facts label. The proposed rule did not include the declaration of the percent daily value for added sugars. More information
|

What's New in Health Disparities? by: Jovonni Spinner, MPH, CHES, Public Health Advisor in FDA's Office of Minority Health
In June 2015, I presented at the Health Disparities, Education, Awareness, Research, and Training (HDEART) workshop at Prairie View A&M University, near Houston. This annual workshop brought together nationally recognized leaders to discuss genomics, communications, bioethics, and other minority health issues, as well as disease-specific health programs, such as cancer, maternal health, and smoking cessation.
We know health disparities exist and minorities fare worse for many health outcomes. That is old news. The workshop promoted an open discussion and offered fresh ideas on bio-psychosocial approaches to address health disparities that will improve health equity.
To continue reading this post, see FDA Voice Blog July 30, 2015
|

The Times They Are a Changin' - And So is FDA, by: Luciana Borio, M.D., Acting Chief Scientist
Recently, at a public meeting of the FDA Science Board, the agency is releasing our progress report, FDA Science Moving Forward, highlighting advances that FDA has made since the Science Board’s 2007 report FDA Science and Mission at Risk. Also at this meeting, a Science Board subcommittee will present its recommendations on FDA’s regulatory science programs, scientific workforce, and collaborations.
As our report FDA Science Moving Forward illustrates, FDA regulatory science programs have made dramatic advances in the last eight years. These advances are critical because, as I’m always reminded, regulatory science underpins virtually every decision we make at FDA.
To continue reading this post, see FDA Voice Blog, July 29, 2015
|

FDA advisory committee meetings are free and open to the public. No prior registration is required to attend. Interested persons may present data, information, or views, orally at the meeting, or in writing, on issues pending before the committee.
Other types of meetings listed may require prior registration and fees.
View FDA's Calendar of Public Meetings page for a complete list of meetings and workshops.
|
- Public Workshop: Medical Device Patient Labeling
Date: September 29, 2015 8:00 am to 5:00 pm
Date: September 30, 2015, 8:00 am to 5:00 pm
Location: FDA White Oak Campus - 10903 New Hampshire Ave, Silver Spring, MD. 20993
Agenda: The purpose of the public workshop is to discuss issues associated with the development and use of medical device patient labeling including content, testing, use, access, human factors, emerging media formats, and promotion and advertising. The Center for Devices and Radiological Health (CDRH) is seeking input into these topics from patients and advocacy groups, academic and professional organizations, industry, standards organizations, and governmental Agencies. Ideas generated during this workshop will help facilitate development or revision of guidances and/or standards for medical device patient labeling.
- Public Meeting: Risk Evaluation and Mitigation Strategies: Understanding and Evaluating Their Impact on the Health Care Delivery System and Patient Access
Date: October 5, 2015, 8:00 am to 5:00pm
Date: October 6, 2015, 8:00 am to 1:00 pm
Location: FDA White Oak Campus - 10903 New Hampshire Ave, Silver Spring, MD. 20993
Agenda: The purpose of the public meeting is to engage in constructive dialogue and information sharing among regulators, the scientific community, the pharmaceutical industry, public health agencies, patients, patient advocates, healthcare system administrators, prescribers, dispensers, hospitals, infusion centers, health informatics experts, third - party payers, distributors, and the general public concerning the impact of REMS on the healthcare delivery system, including the impact on patients and healthcare providers. The discussion will focus on strategies for characterizing and evaluating the impact of REMS on the healthcare delivery system and on patient access to drugs subject to REMS.
Please visit FDA’s Advisory Committee page to obtain advisory committee meeting agendas, briefing materials, and meeting rosters prior to the meetings. You may also visit this page after meetings to obtain transcripts, presentations, and voting results. For additional information on other agency meetings please visit Meetings, Conferences, & Workshops.
Consumer Updates
For previously published Consumer Update articles that are timely and easy-to-read and cover all FDA activities and regulated products. More information
En Español
La información en esta página es para el público en general, y para profesionales y educadores de salud. Esta información puede ser distribuida y publicada sin previa autorización. En Español |

Information about Expanded Access
Expanded access, sometimes called "compassionate use," is the use outside of a clinical trial of an investigational medical product (i.e., one that has not been approved by FDA). FDA is committed to increasing awareness of and knowledge about its expanded access programs and the procedures for obtaining access to human investigational drugs (including biologics) and medical devices. |
Learn about what your physician should do before submitting a request for individual patient expanded access use of an investigational medical product, who may be eligible for expanded access, associated costs, FDA contacts and more. Information for Patients
|
Learn about your responsibilities under the expanded access pathway, how to submit a request for expanded access for an individual patient (including for emergency use), which forms to use, FDA contacts and more. Information for Physicians
|

Expanded Access: Recourse for Patients Out of Options
Welcome to this edition of Medscape Oncology Insights, this 20 minute video joins Harold Burstein, M.D., Ph.D associate professor of Medicine at Harvard Medical School and a medical oncologist in the Breast Oncology Center at Dana-Farber Cancer Institute in Boston and Richard Klein, Director of the Patient Liaison Program in the FDA Office of Health and Constituent Affairs. More information |

Federal judge enters permanent injunction against Wisconsin dietary supplement manufacturers
Three dietary supplement companies, under the same ownership and located in Wautoma, Wisconsin, will not be allowed to manufacture or sell dietary supplement products until FDA has determined that the businesses are in compliance with federal manufacturing regulations and other requirements, according to a federal court order signed Aug. 4, 2015. More information
FDA acts to stop Sacramento tofu and sprout manufacturer from selling adulterated food
On August 3, 2015, U.S. Magistrate Judge Carolyn K. Delaney in the U.S. District Court for the Eastern District of California entered a consent decree against Henh Wong Fresh Produce, a tofu and sprout manufacturer and distributor, and its owner, current manager, and former manager after the U.S. Food and Drug Administration documented multiple violations of federal food safety laws and regulations. The U.S. Department of Justice sought the consent decree on behalf of the FDA. More information |

Raw Produce: Selecting and Serving it Safely
Fruits and vegetables are an important part of a healthy diet. Your local markets carry an amazing variety of fresh fruits and vegetables that are both nutritious and delicious. However, harmful bacteria that may be in the soil or water where produce grows may come in contact with fruits and vegetables and contaminate them. Fresh produce may also become contaminated after it is harvested, such as during preparation or storage. Eating contaminated produce (or fruit and vegetable juices made from contaminated produce) can lead to foodborne illness, often called “food poisoning.” As you enjoy fresh produce and fresh-squeezed fruit and vegetable juices, follow these safe handling tips to help protect yourself and your family. More information and to watch a video on Safe Handling of Raw Produce and Fresh-Squeezed Fruit and Vegetable Juices |

Center for Food Safety and Applied Nutrition
The Center for Food Safety and Applied Nutrition, known as CFSAN, carries out the mission of FDA. The Center provides services to consumers, domestic and foreign industry and other outside groups regarding field programs; agency administrative tasks; scientific analysis and support; and policy, planning and handling of critical issues related to food and cosmetics. More information
Food Facts for You
The Center for Food Safety and Applied Nutrition, known as CFSAN, issues food facts for consumers to keep you and your family safe. More information |

Animal Health Literacy
Animal Health Literacy means timely information for the benefit of all animals and their humans. With continuous communication and outreach, the Center for Veterinary Medicine (CVM) strives to enhance the public trust, promote safe and effective use of the animal health products we regulate, and share our scientific endeavors. CVM provides reliable, science-based information to promote animal and human health. More information and Publicaciones en Español del
Animal and Veterinary Updates
Animal and veterinary updates provide information to keep your pets healthy and safe. |

How to Report a Pet Food Complaint
You can report complaints about a pet food product electronically through the Safety Reporting Portal or you can call your state’s FDA Consumer Complaint Coordinators.
Please have as much of the following information available when submitting your complaint: Consumers often transfer dry pet food into other containers for easier handling. If possible, please save the original packaging until the pet food has been consumed. The packaging contains IMPORTANT information often needed to identify the variety of pet food, the manufacturing plant, and the production date. More information
|
Public Health Education
Tobacco products are harmful, yet widely used, consumer products that are responsible for severe health problems in both users and non-users. These health problems include cancer, lung disease, and heart disease, which often lead to death.

|

Public Education Campaigns
We are investing in a number of public education campaigns, such as The Real Cost, to help educate the public – especially youth – about the dangers of regulated tobacco products. Rooted in science, these efforts are directly linked to our authority to regulate the marketing and sales of tobacco products. More information
Youth and Tobacco
We are working to protect the health of America’s children and ultimately reduce the burden of illness and death caused by tobacco use. More information |
|
Each month, different centers and offices at FDA will host an online session where the public can ask questions to senior FDA officials about a specific topic or just listen in to learn more about FDA. More information
Educational Videos
FDA Food Safety and Modernization Act: An FDA PrimerThe Rulemaking Process: An FDA Primer What is Regulatory Science Taking Acetaminophen Safely |

healthfinder.gov
Welcome to healthfinder.gov, a government Web site where you will find information and tools to help you and those you care about stay healthy. More information /más información |






















.png)












No hay comentarios:
Publicar un comentario