
Volume 24, Number 12—December 2018
Research Letter
Disseminated Spiroplasma apis Infection in Patient with Agammaglobulinemia, France
On This Page
Nicolas Etienne, Laurent Bret, Cécile Le Brun, Hervé Lecuyer, Josquin Moraly, Fanny Lanternier, Olivier Hermine, Agnès Ferroni, Marc Lecuit, Sabine Pereyre1, Laure Beven1, and Olivier Lortholary1
Abstract
We report a disseminated infection caused by Spiroplasma apis, a honeybee pathogen, in a patient in France who had X-linked agammaglobulinemia. Identification was challenging because initial bacterial cultures and direct examination by Gram staining were negative. Unexplained sepsis in patients with agammaglobulinemia warrants specific investigation to identify fastidious bacteria such as Spiroplasma spp.
In February 2017, a 40-year-old man in France who was under immunoglobulin replacement therapy for X-linked agammaglobulinemia experienced a migrating nonitchy papular eruption. Skin biopsy revealed nonspecific lymphocytic dermatitis. One month later, he experienced distal interphalangeal arthritis. Histology of a synovial biopsy sample found a nonspecific lymphocytic infiltrate. Bacterial cultures were negative. In April 2017, febrile arthritis of the left ankle and right knee appeared, along with bilateral wrist and finger tenosynovitis (Technical Appendix Figure). We initiated ceftriaxone (1 g/d) and performed a left ankle joint aspiration 24 hours later. The patient remained febrile with a pulmonary valve murmur of tricuspid regurgitation and purpuric lesions, tenosynovitis in the left ankle and both wrists, and right knee and interphalangeal polyarthritis. Serum C-reactive protein was 136 mg/dL and IgG serum level 9.4 g/L. Transesophageal echography showed slight tricuspid regurgitation. Arthritis worsened, and we performed a right knee joint aspiration. Four of 6 blood cultures were positive after 55 h of incubation at 37°C (Bactec FX, Becton Dickinson, Franklin Lakes, NJ, USA). Results of direct examination by Gram staining of positive blood cultures and real-time multiplex PCR for blood culture identification (FilmArray BCID Panel; bioMérieux, Marcy l’Etoile, France) were negative. Small α-hemolytic areas appeared on subcultures in horse blood agar after 5 days of aerobic culture; results of mass spectrometry performed on these areas were negative. Blood culture–specific PCR targeting Tropheryma whipplei, Bartonella spp., Brucella spp., Coxiella burnetii, and Rickettsia spp. showed negative results.
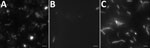
Figure. Direct examination with dark-field microscopy of specimens from a patient with agammaglobulinemia who had Spiroplasma apis infection, France. A) Helical and motile bacteria in blood culture. B) Elongated and coccoid bacteria...
At this stage, we modified antimicrobial therapy to be optimal for Mycoplasma, Chlamydia, deficient streptococci, Campylobacter, and Helicobacterregarding the context of agammaglobulinemia. We introduced amoxicillin/clavulanic acid (12 g/1,200 mg/d) associated with levofloxacin (500 mg 2×/d), leading to apyrexia and negative blood culture after 1 day. Results of testing for Chlamydia trachomatis (by PCR and culture) and Ureaplasma spp. and Mycoplasma hominis (by culture) in urine were negative. We seeded synovial fluid on blood culture, and small colonies developed on horse blood agar. We extracted DNA from the zone of hemolysis using EZ1 DNA tissue kit (QIAGEN, Valencia, CA, USA). We amplified and sequenced the 16S rRNA gene with the primer set 27F/16S1RRB as previously described (1) and performed bacterial identification using phylogenic analysis on the Bioinformatics Bacterial Identification tool (2). The 491-bp amplicon sequence was 100% identical to that of Spiroplasma apis strain ATCC 33834 (GenBank accession no. GU993267). We confirmed identification of S. apis by specific S. apis PCR (3) and culture (on modified SP4 agar) in synovial fluid from left ankle and right knee and in blood culture. Direct examination of synovial fluid and blood culture with dark field microscopy allowed identification of bacteria with a helical morphology and motility evocative of spiroplasmas (Figure). We obtained highly enriched Spiroplasma cultures starting from fluid and blood cultures. We retrospectively found specific PCR for S. apis positive in synovial biopsy from the distal interphalangeal sample. Antimicrobial drug susceptibility testing showed susceptibility to macrolides, tetracyclines, and fluoroquinolones and resistance to rifampin, cotrimoxazole, and penicillin G. Tetracycline and fluoroquinolone MIC values were similar to those reported for Ureaplasma spp.
Patient’s condition gradually improved with 6-week administration of levofloxacin and iterative joint aspirations of the right knee. Because of severe tendinopathy, levofloxacin was stopped after 6 weeks and replaced by azithromycin (500 mg/d) and doxycycline (200 mg 2×/d) for 6 weeks.
Patients with agammaglobulinemia are susceptible to infections, including mycoplasma infections (4). Invasive infections such as endocarditis and septic arthritis have been reported with M. hominis and Ureaplasma urealyticum in hypogammaglobulinemic patients. Of note, our patient contracted mycoplasma infection despite an appropriate gammaglobulin serum level (IgG 9.4 g/L).
Spiroplasmas, arthropod-infecting bacteria of the class Mollicutes, are similar to mycoplasmas. Pathogenic species include S. apis, detected in hives in southwestern France (5). S. apis is associated with the lethal May disease of the European honeybee Apis mellifera. Its prevalence in bee colonies in France was not reported but was recently shown to reach high percentages in other countries (6). Only 3 cases of human infection due to Spiroplasma spp. have been reported: hepatitis in a lung transplant recipient (7), uveitis in a premature neonate (8), and bacteremia in a patient under certolizumab treatment (9). None of these infections correlated with any noticeable insect bite history. In contrast, the patient we report had multiple stings by a flying insect resembling a hornet in August 2016, in the Loiret department of France, leading to cutaneous blebs and transitory rash. This finding raises the possibility that S. apis might infect insects other than honeybees. The insect stings in this patient are a likely gateway of the reported infection.
In summary, clinicians and microbiologists should be aware of fastidious organisms in atypical infections in immunocompromised patients. Our findings indicate a need for prolonged culture on specific agar on all joint fluids in patients with agammaglobulinemia and targeted molecular methods to identify S. apis organisms.
Dr. Etienne is an infectious disease clinician and a clinical immunologist in Necker-Pasteur Center for Infectious Diseases, Paris. His research interest focuses on infections in immunocompromised patients.
Acknowledgment
We thank Anne Laurence Thomi Georgelin, who referred the patient to the Necker-Pasteur Center for Infectious Diseases and Tropical Medicine; Valérie Zeller for the management of septic polyarthritis; and Philippe Lanotte and Marie-Pierre Dubrana, who participated in molecular analysis.
References
- Blanchard A, Bébéar CM. Mycoplasmas of humans. In: Razin S, Herrmann R, editors. Molecular biology and pathogenicity of mycoplasmas. New York: Kluwer Academic/Plenum Publishers; 2002. p. 45–71.
- Mouches C, Bové JM, Tully JG, Rose DL, McCoy RE, Carle-Junca P, et al. Spiroplasma apis, a new species from the honey-bee Apis mellifera. Ann Microbiol (Paris). 1983;134A:383–97.PubMed
Figure
Cite This ArticleOriginal Publication Date: 11/7/2018
1These authors contributed equally to this article.





















.png)












No hay comentarios:
Publicar un comentario