
Drug Discovery of Small Molecules
Phases of Drug Discovery and Development
Small molecule drugs are discovered in a lengthy process that takes several years or even a couple of decades, being split into several phases. A glance at the statistics from 1995 to 2007 shows that the percentage of compounds from these phases that entered clinical trials was about 12.5%.
Kateryna Kon | Shutterstock
Phases of Small Molecule Drug Discovery
The following table shows the different phases of discovering small molecule drugs, in brief.
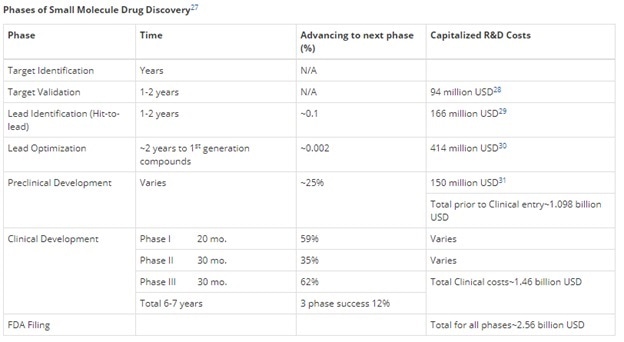
Target Validation
In this phase, it has to be shown that interaction of the drug with the chosen biological target causes the desired effect on biological systems. Biologists and chemists dominate during this phase. Many small molecules available in the market are helpful in achieving this. A compound whose mechanism is known are potentially useful as positive or negative controls in the development of assays, or to screen for unwanted actions of drugs during counter screening.
Some commonly used positive controls are:
- Heat shock protein hsp 60 regulator
- P13 kinase inhibitor
- EFGR kinase inhibitor
- A chymotrypsin/trypsin inhibitor
Small molecules used for counter-screening include:
- some class III antiarrhythmics which are selective hERG channel-mediated potassium current inhibitors
- An hERG channel inhibitor which has antihistaminic effects
- an L-type calcium channel blocker
- a non- selective protein serine/threonine kinase inhibitor
- selective PDE4 inhibitor
Lead Identification (Hit-to-Lead)
The second phase is when novel molecules with promise need to be found, requiring two or three different chemical structures to be investigated simultaneously by methods such as:
- Design and synthesis of new chemical structures inspired by existing patents and literature
- Screening of molecular libraries on a small scale and high throughput
- If X-ray data is available, model development and virtual library screening
This search throws up active compounds or hits which are then narrowed down to a few promising compounds of high value, called leads. These must be tested for validation of their chemical structure by repeating the synthetic process and purification, and their activity must be confirmed by characterization of the biological properties of the material after purification.
At the search stage millions of compounds are screened by high throughput methods, while the number of compounds identified as hits are in the tens or hundreds at most. Of these, the number of molecules confirmed to be leads are less than 20. Finally, lead optimization is carried out in only 2-3 series of lead molecules.
Lead Optimization
A number of desired characteristics must be brought together optimally in a single molecule. These include the right potency, selectivity of action, physical characteristics and in vivo activity. Chemists often need to produce a thousand or ten thousands analogous compounds to get a few compounds in which this balance is found. Thus a wide range of disciplines must be harnessed to find just one or two appropriate preclinical drug candidates.
Preclinical Development
This stage is expensive since every candidate compound is required to have its safety documented in terms of a safety profile, acquired by testing the compounds in weights measured in kilograms. The detailed testing done at this level causes most compounds to fall out of the race here, and it is to replace them that other different chemicals and their derivatives are investigated in the lead optimization phase.
Clinical Development
Once the preclinical trials of the drug are successful, the candidate is ready for clinical testing to assess how safe and effective it is. This takes place in several phases:
- Phase I: the drug is given to healthy humans to check its safety
- Phase II: it is administered to humans in small numbers who have the specific disease
- Phase III: it is given to humans who have the specific disease, on an expanded scale, while administering other drugs as well
- Phase IV: it is approved by the FDA and tested again in more trials to look for any side effects from long-term use
FDA Filing
The company files a New Drug Application (NDA) with detailed supporting data to show that the drug can safely be used for the proposed condition, it has been labeled properly, and can be manufactured to high quality standards.
From Generalized to Personalized Medicine
Any drug development must have the right target to succeed. The use of genetic profiling has uncovered new pathways of fitting the drug to the right target. Especially in cancer treatment, this has transformed the process of discovering new drugs.
Many powerful anti-cancer agents in the past had effects on both healthy and malignant cells, but it was hoped that the greater sensitivity of cancer cells to these effects, due to their markedly higher rate of growth and proliferation, would cause the drug to act selectively.
However, over the last ten or so years, genetically tailored drugs are being developed using the DNA fingerprints of cancer cells. These are expected to show the way cancer cells operate as a result of mutations, which sets them apart from normal cells, and this will help drugs to target them for selective killing.
Some of these small molecule drugs which have been developed over the last ten years are now used to fight:
- Acute promyelocytic leukemia
- Myeloid leukemia
- Non-small cell lung cancer
- ERBB2-positive breast cancer
- Kidney cancer
- Castration-resistant prostate cancer
- Metastatic melanoma
Summary
There are many stages of drug discovery and development which may take ten years or even more in some cases. From start to finish of this process, only one in a thousand drug candidates may be successfully developed to marketable stage. The main focus is on identifying promising molecules and optimizing the drug properties for successful therapy. Preclinical and clinical trials are conducted to evaluate drug safety and efficacy before the regulatory body can approve it.
About SmallMolecules.com
SmallMolecules.com is a product-listing-service website that gives pharmaceutical researchers and academics the ability to browse over 600,000 small molecule products, and easily source them from a variety of manufacturers around the world.
The website allows users to search for products by: CAS#, product name, trivial name, alternative name, catalog number, or chemical formula. Once a user has found their required small molecule product, they can simply click-through to the manufacturer’s website and purchase the product directly from the manufacturer.
The mission of SmallMolecules.com is to make the process of sourcing small molecules as simple and efficient as possible for scientists engaged in life sciences research the world over.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last updated: Oct 3, 2018 at 12:54 PM


































No hay comentarios:
Publicar un comentario