
Antisera to Identify Pathogenic Escherichia coli
Introduction
Diseases Caused by Escherichia coli
Escherichia coli (E. coli) are Gram-negative enterobacteria, of the order Enterobacterales. They do not form spores and typically appear as motile rods, with flagella.
E. coli strains predominate in the human and animal intestine among aerobic commensal bacteria. Though this is a natural biome, some E. coli strains cause human infection like urinary tract infections, diarrhea or gastroenteritis, pus-forming lesions, wound abscesses, septicemia or meningitis in the newborn. E. coli is the most frequently identified cause of acute uncomplicated urinary tract infection. It can also cause animal mastitis, pyometra in bitches, coli granuloma in fowls, and white scours in calves.
Pathogenic E. coli are grouped into four categories on the basis of pathogenic mechanism:
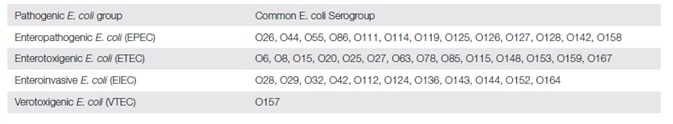
- Enteropathogenic E. coli (EPEC) cause infantile enteritis, which is common especially in the tropics. In these places frequent hospital outbreaks are observed with high mortality, though these are rare in developed nations. The symptoms are usually those of stomach pain, diarrhea and vomiting, with fever.
- Enterotoxigenic E. coli (ETEC) produces its pathogenic effects via a heat-labile or heat-stable enterotoxin (ST and LT respectively), though both may be present. They also adhere to the small bowel epithelial lining by species-specific colonization factors. The toxins produce diarrhea and vomiting, stomach ache and fever.
- Enteroinvasive E. coli (EIEC) cause an illness identical to Shigella dysentery in patients of all agents. Symptoms include diarrhoea (with mucus and blood), stomach ache and fever.
- Enterohemorrhagic E. coli (EHEC) or Verotoxigenic E. coli (VTEC) are so called because they produce one or two Vero cytotoxins VT1 and VT2. Among these, VT1 is very similar to the Shiga toxin of Shigella dysenteriae 1 strains and is therefore called Shiga-like toxin. These organisms produce heterogenous symptoms from light watery diarrhea to severe bloody diarrhea caused by hemorrhagic colitis. The usual symptoms include bloody diarrhea, stomach pain and fever. In children this variant is associated with the specific complication called Hemolytic-Uremic Syndrome (HUS).
The table below shows the most frequently encountered E. coli strains causing diarrhea:
Characterizing E. coli Antigens
E. coli strains have three surface antigenic types, the O or somatic antigen, the K or capsular antigen and the H or flagellar antigen. The organisms are grouped by the heat-stable O antigens. The other two are heat-labile, but the H antigen is used for serotyping the strain. The K antigen formerly included both surface and capsular antigens. It was classified further into L, A and B classes based on how heat changed agglutination, antigenic and antibody binding properties. K antigens are no longer needed for serotyping and the L, A and B designations have been abandoned.
In fact, many so-called K antigens have been found to be nothing of the sort. The K antigen is therefore used only for diagnosis in some circumstances, such as in some toxigenic E. coli which cause animal disease. These have antigenically distinctive pili antigens unlike other E. coli. The pili act to attach the organism and infect the host by anchoring the bacterium to the mucous cells of the intestinal epithelium, and also determine the ability to produce enterotoxin.
E. coli strains associated with swine diarrhea have K88 and 987P antigens. The K99 antigen is found in E. coli isolated from diarrheal samples in lambs and calves. ETEC in humans also show the presence of colonization antigens CFA I, II and E8775, with others being possibly present but not used for the clinical diagnosis in most cases.
Kauffman presented his classification scheme for E. coli using the O antigens in 1947. Today, more than 160 O antigens, 90 K antigens and 50 H antigens are known, and used to serotype pathogenic E. coli.
Preparing and Presenting MAST ASSURE E. coli Antisera
The MAST® ASSURE antisera against pathogenic E. coli are prepared using strains of E. coli that have identified antigenic factors, the strains being standard for this practice.
The sera are inactivated by heat at 56 °C for 30 minutes and any agglutinins that could cause cross-reaction are removed by absorption. The sera are then sterilized by filtration. The Pathogenic E. coli antisera from MAST ASSURE comprise a range of antisera to both O and H antigens of pathogenic E. coli. The antigen determination is performed normally using qualitative or quantitative agglutination on slides or tubes respectively.
The antisera come as 2 or 5 ml vials with droppers, and the preservative used is 0.1% sodium azide. This allows 50 (or 125) slide agglutination procedures or 20 (50) tube agglutination tests.
Preparing E. coli Cultures for Serology
E. coli cross-react and show multiple antigenic relationships with other E. coli as well as other genera of Enterobacterales, especially with Shigella. Therefore, morphologic and biochemical identification of organisms as E. coli is mandatory before doing serological classification testing.
Antisera Products for O and H Antigens from Pathogenic E. coli
The antisera available in the MAST ASSURE polyvalent Pathogenic E. coli O-grouping antisera are given in Table 1 with the product codes.
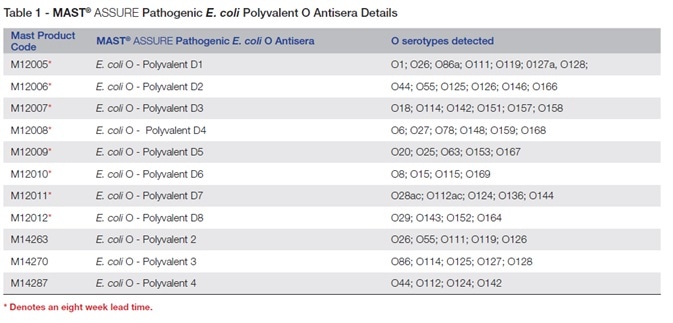
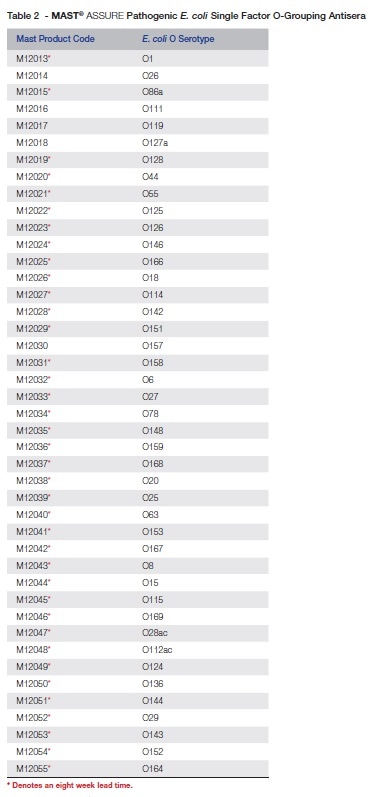
Table 2 shows MAST ASSURE single factor O-grouping antisera for pathogenic E. coli.
Table 3 is a list of MAST ASSURE H-typing antisera against pathogenic E. coli.
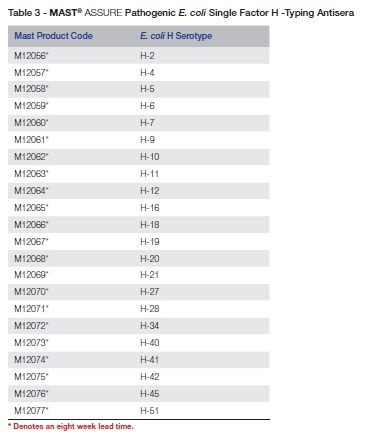
Single factor typing is not feasible for all the E. coli antigens identified, but the large array of sera in the MAST ASSURE range allows most isolates of E. coli to be identified to an acceptable level of certainty.
Polyvalent O-antisera must be used first to narrow the range of O groups of the pathogenic E. coli isolate, and then specific grouping sera are used to identify it, as Figure 1 shows.
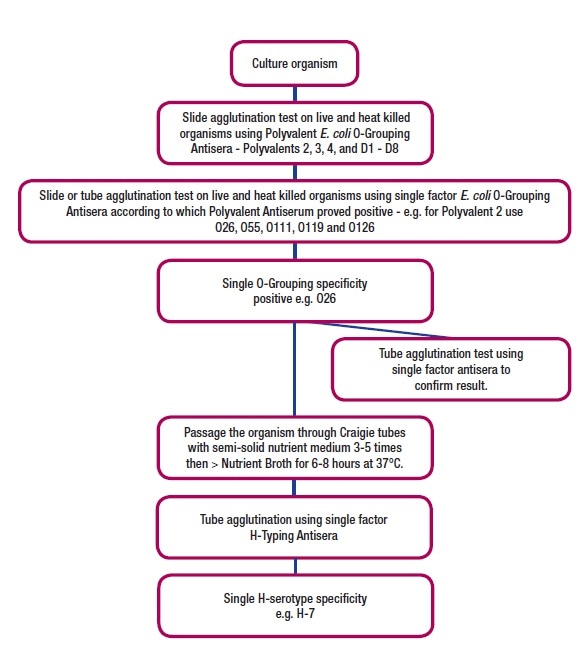
Figure 1: Summary of Pathogenic E. coli O-Grouping and H-Serotyping Procedures.
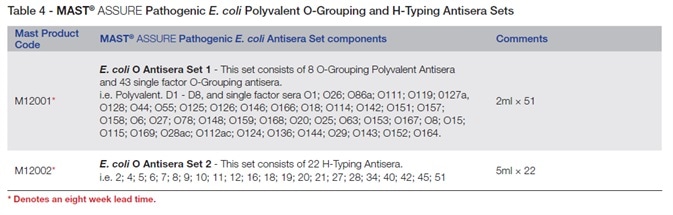
MAST ASSURE pathogenic E. coli antisera may also be obtained as easy-to-use sets as Table 4 shows, with separate bottles of specific antiserum.
About Mast Group
Mast is an independent world class manufacturer and supplier of diagnostic products for clinical, industrial and veterinary testing.
The parent company, Mast Group Ltd., has corporate headquarters in Bootle near Liverpool, UK. Mast also has Subsidiary companies in Reinfeld, Germany (Mast Diagnostica GmbH) and Amiens, France (Mast Diagnostic).
Manufacturing of the core range of microbiology products takes place in Bootle while other infectious disease and autoimmune diagnostics, using a variety of technologies, are produced in Reinfeld.
Mast offers its extensive product range through its own companies plus a global network of Distributors, for example, Mast has a long standing association with Davies Diagnostics in Johannesburg, South Africa.
Mast also supplies on a country by country basis, other quality diagnostic products from several internationally renowned manufacturers.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last updated: Oct 18, 2018 at 10:01 AM


































No hay comentarios:
Publicar un comentario