
Use of the Livecyte® System to Characterise Heterogeneous Primary Prostate Cancer Epithelial Cells
Introduction
Today, patient-derived primary cell cultures are increasingly being preferred in preclinical studies, because these cell lines not only provide a better model for tumour heterogeneity but also represent inter- and intra-patient diversity much more accurately than conventional ones.
Targeting specific sub-populations of cell types will help develop effective treatments, which means combination treatments could probably be more effective than monotherapies. In this article, heterogeneity within primary prostate epithelial cell cultures harvested from a patient’s tumour tissue was imaged and characterised using the non-perturbing imaging modality available on the Livecyte Cell Imaging and Analysis system from Phasefocus™.
Methods
First, cell populations of primary prostate cancer cell cultures were divided into two sub-populations on the basis of their collagen adherence properties; Committed Basal (CB) and Transit Amplifying (TA) cells.
Flow cytometry and immunofluorescence were initially used to investigate the TA and CB cell phenotypes. In addition, the sub-populations were examined and characterised with Livecyte’s label-free Quantitative Phase Imaging (QPI) modality and the Livecyte Cell Analysis Toolbox (CAT) software.
To find out whether both sub-populations can be effectively identified within a mixed population (whole population – WP), the exclusive phase metrics distinguished from the isolated cell populations were applied to this heterogeneous WP culture of primary prostate cells.
Results
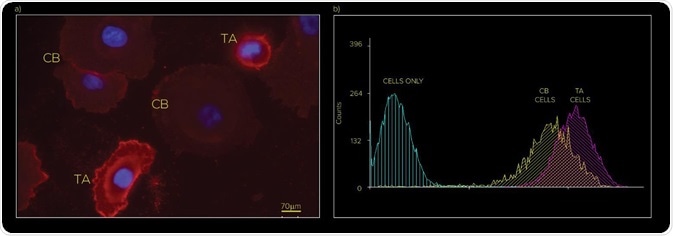
As illustrated in the fluorescence image in Figure 1(a), CB and TA cells show a slight difference in α2β1 integrin expression, but as depicted by flow cytometry in Figure 1(b) this difference is not sufficiently clear to distinctly separate the cell populations.
Figure 1. (a) Immunofluorescence and (b) Flow Cytometry analysis of TA and CB subpopulations based on expression of CD49b (α2β1 integrin)
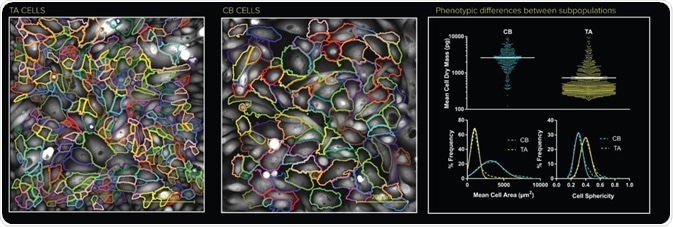
QPI time lapse was obtained for both the CB cells and the TA cells. Figure 2 shows single frames for each subpopulation (CB and TA). Using the Livecyte analysis software, each individual cell was segmented for every frame within the time lapse and the multiple metrics were extracted, as shown in Figure 2.
Segmentation of individual cells makes it possible to extract multi-parametric data from one population. In this analysis, dynamic and morphological phenotypes for each cell were obtained at each time point.
Figure 2. Individual cell segmentation of the TA and the CB populations. Metrics calculated from the Livecyte’s Cell Analysis Toolbox (CAT) relating to each population displays distinct phenotypes.
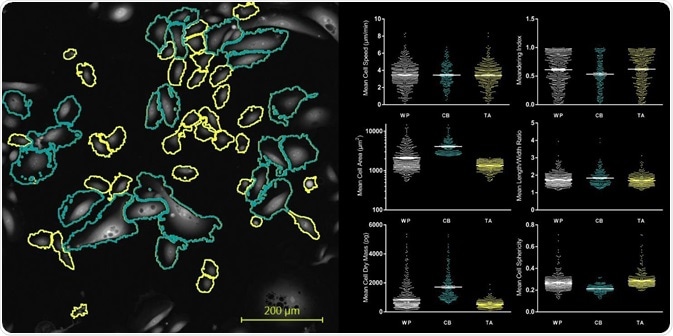
Figure 3 shows a single frame from the WP time lapse as well as the extracted metrics detecting both subpopulations within the mixed culture.
Obviously, CB cells possess a larger cell area, a slightly reduced cell sphericity, and an overall larger dry mass, demonstrating how cells can be divided into TA, CB, and WP groups according to the distribution of area, speed, dry mass, ellipticity, sphericity, and meandering index.
Figure 3. Individual cell segmentation of the TA and CB subpopulation within a heterogeneous culture. The suite of metrics extracted using the Livecyte’s Cell Analysis Toolbox (CAT) software illustrate distinct phenotypic differences between the subpopulations.
One can clearly see the segmented images of each population. Both visually and from the extracted data, it is obvious that the CB cells indeed have a lower sphericity and a much larger area. Volumetric data can be extracted due to the quantitative nature of the QPI method.
The CB cells, in this case, clearly show a higher dry mass. A greater discrepancy is also observed in the cell region of the CB population. The data clearly demonstrates that the individual cell analysis corresponds with the results obtained from flow cytometry analysis (Figure 1b).
Conclusion
This article shows how the Livecyte system can be used for imaging heterogeneous cell populations and combining the multi-parametric data extracted to automatically categorise and differentiate sub-populations within intricate co-cultures.
Thanks to the non-perturbing nature of the QPI technique, primary cell cultures can be reliably imaged without the ambiguity in cell behaviour caused by the inclusion of fluorescent labels or photo-induced behaviour.
Being a highly robust tool, the Livecyte Cell Imaging and Analysis system can be used for developing combination therapy drugs for heterogeneous tumour cell cultures derived from patients.
Acknowledgements
Phasefocus wish to acknowledge Prof. Norman. Maitland, Dr Fiona Frame and Dr Amanda Noble from the Cancer Research Unit, University of York (https://www.york.ac.uk/biology/research-groups/cru/) for the work they have contributed to this application note.
About PhaseFocus
Phasefocus provides a range of products and services based on its proprietary Ptychographic Quantitative Phase Imaging (QPI) technology, pertinent to a wide range of analytical applications requiring reliable and robust image capture and data handling.
The company’s flagship product Livecyte® addresses the limitations of phase contrast imaging, offering a revolutionary new way to study cell morphology and dynamic behaviour at the individual cell level, with unprecedented accuracy.
The Phasefocus technology permits capture of information rich phase data at multiple wavelengths enabling observation and analysis of materials, processes and products at nano scale level.
With broad spectrum appeal, this innovative technology has potential for use in diverse markets ranging from life science and healthcare to engineering, metrology and more.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last updated: Sep 28, 2017 at 4:28 AM






















.png)











No hay comentarios:
Publicar un comentario