
Elements That Keep Us Alive Also Give Color to Fireworks
Looking up at the night sky this Fourth of July, you might wonder what gives fireworks their vivid colors. The bright hues result from chemical elements that are also essential for life. Chemists and other researchers have been uncovering their roles in a range of important biological processes.
By mass, about 96 percent of our bodies are made of four key elements: oxygen (65 percent), carbon (18.5 percent), hydrogen (9.5 percent) and nitrogen (3.3 percent). These elements do not give color to fireworks, but they are found in our body’s most abundant and important molecules, including water, proteins and DNA.
A dozen or so other elements—mostly metals—make up the remaining 4 percent. Present in minuscule amounts, these elements are involved in everything from transporting oxygen and releasing hormones to regulating blood pressure and maintaining bone strength. They also add a burst of color when put in to a fireworks recipe. Here are several examples.

Calcium is the most plentiful mineral in the human body and is needed to make orange fireworks. It’s crucial for a range of bodily functions including bone and teeth maintenance, muscle contraction, hormone secretion, blood clotting and heartbeat regulation.
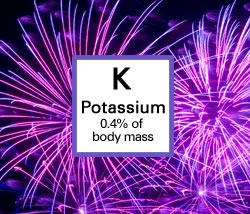
Potassium, which helps create purple fireworks, also plays a role in managing heart rhythm. In addition, it balances water and mineral content in the body, helps to build muscle, and controls blood pressure.

Copper, found in bright blue fireworks, is the sidekick that some proteins need to do their jobs. For example, the protein that makes the body’s energy-carrying ATP molecules requires copper to function. Copper is also necessary to form collagen, the most abundant protein in humans and the main component of connective tissue.
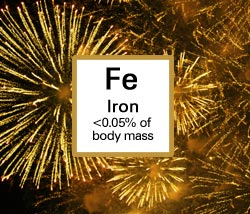
Iron, used to make gold fireworks, is vital to immune function, energy production and oxygen transport in the body. Hemoglobin, the protein that gives blood its red color, needs iron to carry oxygen from the lungs to the rest of the body.

Lithium helps produce red fireworks and is thought to affect the release of the chemical messenger serotonin. Lithium has been used for decades to treat mood disorders.
See an infographic on How Elements in Fireworks Make the Human Body Work  . For details about other elements’ roles in the body, see Metals in Health and Disease.
. For details about other elements’ roles in the body, see Metals in Health and Disease.






















.png)












No hay comentarios:
Publicar un comentario