
Volume 24, Number 4—April 2018
Research
Phenotypic and Genotypic Characterization of Enterobacteriaceae Producing Oxacillinase-48–Like Carbapenemases, United States
On This Page
Joseph D. Lutgring , Wenming Zhu, Tom J.B. de Man, Johannetsy J. Avillan, Karen F. Anderson, David R. Lonsway, Lori A. Rowe, Dhwani Batra, J. Kamile Rasheed, and Brandi M. Limbago
, Wenming Zhu, Tom J.B. de Man, Johannetsy J. Avillan, Karen F. Anderson, David R. Lonsway, Lori A. Rowe, Dhwani Batra, J. Kamile Rasheed, and Brandi M. Limbago
Abstract
Oxacillinase (OXA)–48–like carbapenemases remain relatively uncommon in the United States. We performed phenotypic and genotypic characterization of 30 Enterobacteriaceae producing OXA-48–like carbapenemases that were recovered from patients during 2010–2014. Isolates were collected from 12 states and not associated with outbreaks, although we could not exclude limited local transmission. The alleles β-lactamase OXA-181 (blaOXA-181) (43%), blaOXA-232 (33%), and blaOXA-48 (23%) were found. All isolates were resistant to ertapenem and showed positive results for the ertapenem and meropenem modified Hodge test and the modified carbapenem inactivation method; 73% showed a positive result for the Carba Nordmann–Poirel test. Whole-genome sequencing identified extended-spectrum β-lactamase genes in 93% of isolates. In all blaOXA-232isolates, the gene was on a ColKP3 plasmid. A total of 12 of 13 isolates harboring blaOXA-181 contained the insertion sequence ΔISEcp1. In all isolates with blaOXA-48, the gene was located on a TN1999 transposon; these isolates also carried IncL/M plasmids.
The prevalence of carbapenem-resistant Enterobacteriaceae (CRE) has been increasing in the United States since 2000 (1,2). This finding is problematic because treatment options for CRE infection are limited, and these infections are associated with a higher mortality rate than are infections with carbapenem-susceptible Enterobacteriaceae (3). Enterobacteriaceae might be resistant to carbapenems by a variety of mechanisms, the most concerning of which is production of carbapenemases (4). Although the Klebsiella pneumoniae carbapenemase is the most common carbapenemase reported in the United States, there have been reports of several other carbapenemases including the metallo-β-lactamases and, more recently, oxacillinase (OXA)–48–like carbapenemases (1,5–10).
OXA-48 is a member of the ambler class D β-lactamase family, first described in a K. pneumoniae isolate from Turkey in 2004 (11). The OXA-48 enzyme hydrolyzes penicillins efficiently, carbapenems slowly, and extended-spectrum cephalosporins poorly; it is not inhibited by tazobactam, sulbactam, or clavulanic acid (12). Since the initial report, OXA-48 has established reservoirs in Turkey, the Middle East, countries in North Africa, and throughout Europe (12). These reservoirs have been reported in multiple Enterobacteriaceae species in addition to K. pneumoniae, including Citrobacter freundii, Enterobacter cloacae, Escherichia coli, K. oxytoca, Serratia marcescens, and Providencia rettgeri (12). In addition to OXA-48, several variants with similar enzymatic profiles have been described, including OXA-162, -181, -204, -232, -244, -245, -370, -436, -438, and -484; each variant differs from OXA-48 by only a few amino acids (12–16). Other variants that do not hydrolyze carbapenems have also been described, including OXA-163, -247, and -405 (13,17,18).
The first description of isolates with β-lactamase OXA-48–like (blaOXA-48−like) genes in the United States was from a surveillance study in 2013, which incidentally reported 2 K. pneumoniae isolates (6). This description was followed shortly afterward by a report of 2 clinical K. pneumoniae isolates with blaOXA-48−like genes in patients from 1 institution in Virginia who had traveled internationally (7). More recently, CRE with blaOXA-232 genes have been isolated in the United States (8). The Centers for Disease Control and Prevention (CDC) has collected multiple isolates harboring blaOXA-48−like genes from patients in the United States (19). We report the genotypic and phenotypic characterization of those isolates.
Collection of Isolates
Isolates are submitted to CDC for antimicrobial susceptibility testing (AST) for many reasons, including outbreak response, AST confirmation, and surveillance studies. Surveillance studies include the Multi-Site Gram-Negative Surveillance Initiative, which is part of the Emerging Infections Program, and the Sentinel Study (5,20). All Enterobacteriaceae isolates received for AST at CDC during June 1, 2010–October 31, 2012, with reduced susceptibility to carbapenems (MIC >1 μg/mL for any carbapenem), a positive modified Hodge test result, and a PCR-negative result for bla K. pneumoniae carbapenemase were retrospectively screened for blaOXA-48−like genes (n = 115). During November 1, 2012–September 30, 2014, all Enterobacteriaceae received at CDC were routinely tested for blaOXA-48−like genes by real-time PCR (n = 1,399). Submitting institutions were characterized by state and US Department of Health and Human Services (HHS) region (https://www.hhs.gov/ash/about-ash/regional-offices/index.html).
Phenotypic Characterization of Isolates
We performed reference broth microdilution AST on all isolates by using in-house prepared frozen panels that included carbapenems, cephalosporins, aztreonam, penicillins, quinolones, trimethoprim/sulfamethoxazole, aminoglycosides, chloramphenicol, tetracyclines, tigecycline, polymyxin B, and colistin (21,22). The modified Hodge test, Carba Nordmann–Poirel test, and the modified carbapenem inactivation method (mCIM) were performed on all blaOXA-48−like isolates according to Clinical and Laboratory Standards Institute guidelines (22). We confirmed species identification by using the Biotyper 3.1 MALDI System (Bruker Daltronics, Billerica, MA, USA).
Genotypic Characterization of Isolates
The PCR for blaOXA-48−like genes was developed at CDC and detects blaOXA-48, blaOXA-162, blaOXA-163, blaOXA-181, blaOXA-204, blaOXA-232, blaOXA-244, blaOXA-245, blaOXA-247, blaOXA-370, blaOXA-405, blaOXA-438, blaOXA-484, and blaOXA-505 by using 2 sets of blaOXA-48−like primers/probes and a bacterial 16S rRNA gene as an endogenous control for lysate validation and PCR amplification (Table 1). We extracted DNA by using the thermal/sodium hydroxide method for preparation of bacterial cell lysates (23). Cycling conditions were a 3-min enzyme activation step at 95°C, followed by 40 cycles for 3 s at 95°C, and a final step for 30 s at 60°C (24).We characterized all isolates positive for blaOXA-48−like genes by using whole-genome sequencing (WGS). We extracted DNA by using the Maxwell 16 Cell Low Elution Volume DNA Purification Kit (Promega, Madison, WI, USA) and fragmented input genomic DNA (gDNA) with an absorbance ratio of 1.8–2.0 to ≈800 bp by an using an ultrasonic fragmentation system (Covaris, Woburn, MA, USA). We prepared libraries by using the Ovation Ultralow DR Multiplex System 1–96 Kit (Nugen Technologies, Inc., San Carlos, CA, USA), then multiplexed, and sequenced with MiSeq V2.0 (Illumina, San Diego, CA, USA). We filtered raw Illumina sequencing reads for quality (average >Q20) and discarded trimmed reads >50 bp from the dataset by using SolexaQA version 3.1 (25). We then assembled clean reads into contigs by using SPAdes version 3.1.0 and 4 k-mer sizes (k = 41, 79, 85, and 97) (26). Afterward, we mapped trimmed reads back to each assembled genome by using the Burrows-Wheeler Alignment tool for minor contig error correction (27).
We randomly selected K. pneumoniae isolates 1, 11, and 23, encoding blaOXA-181, blaOXA-232, and blaOXA-48, respectively, as internal reference strains and sequenced them by using Single Molecule Real-Time Technology (Pacific Biosciences, Menlo Park, CA, USA) in addition to Illumina sequencing (Table 2). We extracted and purified gDNA by using the MasterPure Complete DNA and RNA Kit (Epicenter, Madison, WI, USA), according to the manufacturer’s recommended protocol. We generated 10-kb libraries by using the SMRTbell Template Prep Kit 1.0 (Pacific Biosciences) and sequenced libraries by using C4v2 Chemistry on the RSII Instrument (Pacific Biosciences). We assembled data by using Hierarchical Genome-Assembly Process version 3.0 (Pacific Biosciences) and generated clean consensus sequences by using Quiver (28).
We deposited all raw sequencing reads, Pacific Biosciences assemblies, and MIC results in GenBank under BioProject PRJNA296771. We determined multilocus sequence types for each specimen by mapping clean Illumina reads to allele sequences (http://www.pubmlst.org) by using SRST2 software (Illumina) (29). We described antimicrobial resistance genotype profiles from assembled Illumina and Pacific Biosciences contigs by using SSTAR V1.0 (30) in combination with the ARG-ANNOT (31) and ResFinder (32) repositories.
We used the PlasmidFinder database (http://www.genomicepidemiology.org/) to detect plasmid replicon sequences among Illumina and Pacific Biosciences contigs to estimate the plasmid composition of each isolate (33). In addition, we predicted insertion sequences that might be associated with spread of antimicrobial resistance genes by using ISfinder (34). For isolates with blaOXA-48, we estimated the copy number of IS1R insertion sequences for determining Tn1999 variants by using blastn (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and SPAdes K-mer coverage output (26,34–36). The clonality of our plasmids was also assessed, as was the location of blaOXA-48−like genes (Technical Appendix[PDF - 416 KB - 4 pages]). Because of a cluster of isolates from 1 state in this study, a phylogenetic tree and single-nucleotide polymorphism (SNP) tree matrix were produced by using RAxML version 8 (37) (Technical Appendix[PDF - 416 KB - 4 pages]).
Transformation Experiments
We randomly transformed 10 selected isolates (3 with blaOXA-48, 4 with blaOXA-181, and 3 with blaOXA-232) for transformation experiments to better characterize plasmids harboring blaOXA-48−like genes. We subcultured parent isolates on trypticase soy agar containing 5% sheep blood, placed them in 50 mL of tryptic soy broth containing ertapenem (1 μg/mL), and incubated them overnight at 35°C. We extracted plasmid DNA by using Plasmid Midi Kits (QIAGEN, Valencia, CA, USA), according to the manufacturer’s protocol. We digested intact plasmid DNA and gDNA with HindIII (New England Biolabs, Ipswich, MA, USA) and separated this DNA by electrophoresis on a 0.9% agarose gel.
We transformed 500 ng of plasmid DNA from each isolate into E. coli DH10B cells (Invitrogen, Carlsbad, CA, USA) by electroporation and incubated at 35°C for 2 h. Potential transformants were plated on Luria–Bertani agar containing ertapenem (1 μg/mL) and incubated overnight at 35°C. Four colonies from each transformant plate were screened for blaOXA-48−like genes by using PCR. Transformant plasmid DNA was digested and separated by gel electrophoresis along with digested parent plasmid DNA to ensure that transformant plasmids were also present in parental cells.
We characterized confirmed transformants by using AST, the modified Hodge test, and WGS with MiSeq V2.0 (Illumina), as described previously. Trimmed reads from transformants were mapped to the genome sequence of E. coli K12, substrain DH10B (GenBank accession no. NC_010473.1), by using Bowtie 2 software (38,39). Unmapped reads were extracted by using bam2fastq (https://gsl.hudsonalpha.org/information/software/bam2fastq) and were considered to represent plasmid DNA harboring blaOXA-48−like genes (https://gsl.hudsonalpha.org/information/software/bam2fastq). We subsequently assembled these unmapped reads by using SPAdes software and screened generated contigs for antimicrobial drug resistance genes by using SSTAR V1.0 and for plasmid replicon sequences by using the PlasmidFinder database (26,30,33).
Epidemiology of Isolates
We included all 30 US isolates in our collection that were positive for a blaOXA-48−like carbapenemase gene in this study. Isolates were submitted from patients in 12 states representing 8 HHS regions: one from region 1, two from region 2, four from region 3, three from region 4, eight from region 5, three from region 6, eight from region 9, and one from region 10. K. pneumoniaepredominated (n = 27, 90%), although single isolates of K. ozaenae, Enterobacter aerogenes, and E. coli (n = 1 each, 3%) were also found. Isolates were collected from a variety of sources: urine (n = 15, 50%), respiratory samples (n = 10, 33%), peritoneal fluids (n = 2, 7%), wounds (n = 1, 3%), rectal swab specimens (n = 1, 3%), and unknown sources (n = 1, 3%) (Table 2).
Phenotypic Characterization of Isolates
All submitted isolates with a blaOXA-48−like carbapenemase gene showed resistance to ertapenem and all penicillins tested (including those with β-lactamase inhibitors). Most showed intermediate resistance or resistance to imipenem (n = 30, 100%), meropenem (n = 28, 93%), doripenem (n = 28, 93%), ceftriaxone (n = 29, 97%), ceftazidime (n = 27, 90%), and cefepime (n = 28, 93%). In addition, all isolates had a colistin MIC <2 μg/mL (Table 3). Results for the ertapenem modified Hodge test, meropenem modified Hodge test, and mCIM were positive for all isolates harboring blaOXA-48−like genes. The Carba Nordmann–Poirel test result was positive for 73% of isolates, indeterminate in 13%, and negative in 13% (Table 2).
Transformation Experiments
We purified plasmid DNA from 10 isolates (3 with blaOXA-48, 4 with blaOXA-181, and 3 with blaOXA-232) for transformation into E. coli DH10B. Transformants were obtained for each preparation from strains harboring blaOXA-48 and blaOXA-232, as confirmed by PCR and phenotypic and genotypic characterization of each transformant (Table 4). Transformation was unsuccessful for all DNA preparations from strains with blaOXA-181 (isolates 1, 2, 26, and 27).
When we compared transformants with parent strains, most of which harbored multiple plasmids and numerous resistance genes, transformants were confirmed to carry only 1 plasmid and typically showed greater susceptibility to extended-spectrum cephalosporins but retained resistance to >1 carbapenem. As confirmed by WGS, we found that ESBL genes were not typically present on the same plasmid as blaOXA-48−like genes; only 1 transformant (23T) carried a plasmid harboring blaCTX-M-14b on the IncL/M plasmid carrying blaOXA-48. Similar to the parent strain, strain 23T showed increased MICs to cephalosporins and carbapenems, although the carbapenem MICs were lower than both the parent strain and other transformants carrying only an OXA-48−like carbapenemase (Table 4). None of the plasmids harboring blaOXA-48−like genes encoded additional carbapenemases.
Genotypic Characterization of Isolates
We confirmed by using WGS the presence of blaOXA-48−like genes in every isolate, including the alleles blaOXA-48 (n = 7, 23%), blaOXA-181 (43%), and blaOXA-232 (33%). The gene blaNDM was identified in 5 isolates with blaOXA-232. Nearly all isolates (93%) contained >1 ESBL gene, including blaSHV-12, blaCTX-M-14b, and blaCTX-M-15 (Table 2). We also found aminoglycoside, fluoroquinolone, sulfonamide, trimethoprim, tetracycline, chloramphenicol, macrolide, and fosfomycin resistance genes. Multilocus sequence typing of 27 K. pneumoniae isolates showed ST34 (n = 7), ST14 (n = 7), ST16 (n = 3), ST43 (n = 3), and ST101 (n = 3) to be most common in this collection (Table 2).
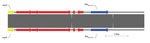
Figure 1. Sequence structure of 2 β-lactamase OXA-232 (blaOXA-232) plasmids tested during phenotypic and genotypic characterization of Enterobacteriaceae producing OXA-48–like carbapenemases, United States. Top plasmid is from isolate 11 in this study (pColKP3_DHQP1300920)...
Isolates 1, 11, and 23 (carrying blaOXA-181, blaOXA-232, and blaOXA-48, respectively) were randomly chosen for Pacific Biosciences WGS in addition to Illumina WGS. Isolate 1 had 2 plasmids and encoded 20 antimicrobial drug resistance genes, including 3 chromosomal copies of the ESBL CTX-M-15; blaOXA-181 was also chromosomally located, with an upstream ΔISEcp1 insertion sequence. The ΔISEcp1 insertion sequence has been described elsewhere (40–42). Isolate 11 had 4 plasmids and encoded 34 antimicrobial drug resistance genes, including plasmid-mediated blaCTX-M-15 and blaNDM-1genes. The blaOXA-232 allele in isolate 11 was found on a ColKP3 plasmid (plasmid size 6,139 bp, G + C content 52.17%); upstream of blaOXA-232, there was a ΔISEcp1 insertion sequence. The sequence of this plasmid (pColKP3_DHQP1300920) has been deposited in GenBank under accession no. CP016920.1. pColKP3_DHQP1300920 was most similar to a ColKP3 plasmid previously deposited under GenBank accession no. JX423831 (100% query coverage, 99% sequence similarity) (Figure 1) (43).

Figure 2. Sequence structure of 2 β-lactamase OXA-48 (blaOXA-48) plasmids tested during phenotypic and genotypic characterization of Enterobacteriaceaeproducing OXA-48–like carbapenemases, United States. Top plasmid is from isolate 23 in this study (pIncL_M_DHQP1400954)...
Isolate 23 had 3 plasmids and encoded 16 antimicrobial drug resistance genes, including 2 ESBLs (plasmid-mediated CTX-M-14b and CTX-M-15). blaOXA-48 was present on an IncL/M plasmid (plasmid size 72,093 bp, G + C content 50.55%). This plasmid contained 89 open reading frames, including those for several antimicrobial drug resistance genes (blaCTX-M-14b, [streptomycin] strA, strB, and [aminoglycoside] aph(3′)-VIb), in addition to blaOXA-48, which appears to have been inserted into the plasmid by transposon Tn1999.2 (GenBank accession no. JN714122). The sequence of this plasmid (pIncL_M_DHQP1400954) has been deposited in GenBank under accession no. CP016927.1. This plasmid, pIncL_M_DHQP1400954 was most similar to pOXA48-Pm (GenBank accession no. KP025948) (95% query coverage, 99% sequence similarity) (Figure 2) (44).
![Thumbnail of Phylogenetic tree of 7 sequence type 34 Klebsiella pneumoniae isolates tested during phenotypic and genotypic characterization of Enterobacteriaceae producing oxacillinase-48–like carbapenemases, United States. Genetic diversity ranged from 1 to 33 high-quality single-nucleotide polymorphisms that were called in an ≈5 Mb core genome, which equals ≈90% of the reference genome size (isolate 1 sequenced by using Pacific Biosciences [Menlo Park, CA, USA], technology). Scale bar indicate](https://wwwnc.cdc.gov/eid/images/17-1377-F3-tn.jpg)
Figure 3. Phylogenetic tree of 7 sequence type 34 Klebsiella pneumoniae isolates tested during phenotypic and genotypic characterization of Enterobacteriaceaeproducing oxacillinase-48–like carbapenemases, United States. Genetic diversity ranged from 1 to 33 high-quality...
We identified no SNPs when we compared Illumina and Pacific Biosciences genome sequences for the same isolate for isolates 1, 11, and 23. This finding indicates that Pacific Biosciences sequences can be used as a mapping reference. We compared Illumina sequence data for the remaining clinical isolates, which were not subjected to Pacific Biosciences sequencing, against the Pacific Biosciences genomes according to blaOXA-48−like allele. For all 10 isolates containing blaOXA-232, the gene was co-located with the ColKP3 replicon gene and a ΔISEcp1 upstream insertion sequence (upstream of blaOXA-232) on an ≈6 kb contig. Pacific Biosciences sequence analysis of isolate 23 confirmed the presence of blaOXA-48 on transposon Tn1999.2; blaOXA-48 was found on a variant of transposon Tn1999 in all instances. In 3 isolates (23, 28, and 29), coverage of the IS1R insertion sequence was similar to the overall assembly coverage suggestive of the Tn1999.2 variant identified in isolate 23 by Pacific Biosciences sequencing. However, in 4 isolates (14, 21, 25, and 30), coverage of the IS1R insertion sequence was much higher than the overall assembly coverage, indicating multiple occurrences of this locus, suggestive of a different Tn1999 variant. Of the 13 isolates containing blaOXA-181, 12 had an upstream insertion sequence ΔISEcp1. In isolate 1, which was sequenced by using Pacific Biosciences technology, blaOXA-181 was confirmed as being chromosomally located. Finally, given the geographic association of several isolates carrying blaOXA-181, we created a phylogenetic tree and SNP matrix table for the 7 K. pneumoniae isolates from 1 state in HHS region 9 (Table 5; Figure 3).
The increasing prevalence of CRE in the United States poses a challenge to patients, clinicians, and public health. The diversity of carbapenemases, including the OXA-48−like enzymes reported in this study, is an ongoing diagnostic challenge to clinical microbiology laboratories because of the variety of phenotypes displayed by isolates producing different, and sometimes multiple, carbapenemases. OXA-48 has been described as the phantom menace because of its subtle phenotype in the absence of co-resistance mechanisms (12).
In this study, all isolates with blaOXA-48–like genes showed resistance to ertapenem, and most showed intermediate resistance or resistance to meropenem, ceftriaxone, ceftazidime, and cefepime. Three tests for carbapenemase production were performed on the isolates in this study. The modified Hodge test, performed for ertapenem or meropenem, and the mCIM showed positive results for all isolates with blaOXA-48−like genes. The Carba Nordmann–Poirel test showed positive results for 73% of all isolates, which is consistent with other studies that have shown that this test had a sensitivity of 72%–76% for OXA-48−like carbapenemase producers (45,46). All isolates in this study would be identified as CRE by the current CDC and Council of State and Territorial Epidemiologists definitions (https://www.cdc.gov/hai/organisms/cre/definition.html) (47).
The 10 isolates that harbored blaOXA-232 were all found on a small ColKP3 plasmid, and this association has been reported by Potron et al. (43). Likewise, the 7 isolates producing OXA-48 carried blaOXA-48 on a similar genetic environment to those reported (44,48,49). Isolate 23, which was sequenced by using Illumina and Pacific Biosciences technology, harbored blaOXA-48 on an IncL/M plasmid. The other 6 isolates, which were sequenced only by using Illumina technology, all had the IncL/M replicon gene. In addition, blaOXA-48 was always associated with a variant of transposon TN1999, as discerned on the basis of the copy number of IS1R insertion sequences (36). Because these IS1R sequences are identical and duplicated, Illumina technology often fails to assemble these as separate loci but instead produces a single locus with high coverage. Comparing coverage of the IS1R insertion sequence to the overall coverage of the assembly sequence enabled us to estimate the presence of the TN1999 variant by using isolate 23 as the reference. In 12 of 13 isolates with blaOXA-181, we found an upstream ΔISEcp1 element inserted upstream of the blaOXA-181cassette. blaOXA-181 is often associated with ISEcp1, which might facilitate its spread (50).
The transformation experiment helped to clarify our understanding of the plasmids harboring blaOXA-48−like genes. Transformation experiments were successful for each of the parent strains carrying blaOXA-48 or blaOXA-232. Carbapenem and penicillin MICs were not different between the parent and transformant, but transformant MICs were comparatively lower for cephalosporins and aminoglycosides. This finding supports the genotypic data, which indicated that ESBL genes and other β-lactamase genes did not cotransfer with the plasmid encoding blaOXA-48−like genes. One transformant (23T) did not have decreased cephalosporin MICs when compared with its parental strain, which is consistent with Pacific Biosciences sequencing of this isolate, which showed blaCTX-M-14b to be on the same IncL/M plasmid as blaOXA-48. The unsuccessful transformation attempts of blaOXA-181–containing strains 1, 2, 26, and 27 was explained by WGS evidence that blaOXA-181 was chromosomally located in isolate 1.
We also detected a possible reservoir of isolates with blaOXA-48−like genes in the United States. Among the 13 isolates with blaOXA-181, 8 were from 1 state in HHS region 9 and contained blaCTX-M-15, blaSHV-26, and ampH. Seven of these isolates were K. pneumoniae belonging to ST34, and 5 were collected during June 2010−May 2011 (Tables 2,5; Figure 3).
This study had several limitations. The collection of isolates in this study might not be representative of all isolates with blaOXA-48−like genes in the United States. There is also a reporting bias because only isolates sent to CDC were included. CDC receives isolates as part of outbreak investigations, surveillance studies, and to confirm AST results, but there is no national requirement to submit carbapenemase-producing isolates. Thus, unusually resistant isolates are more likely to be sent to the CDC and included in this study. Also, no prevalence rates of Enterobacteriaceae with blaOXA-48−like genes in the United States can be inferred because there is not an evaluable denominator. In addition, almost all the isolates we studied were clinical isolates; colonizing isolates might have different phenotypic characteristics.
Another limitation is that the 10 isolates selected for the transformation experiment and the 3 isolates selected for Pacific Biosciences sequencing might not have been representative of the other isolates in this collection. Ideally, all isolates would have been sequenced by using Pacific Biosciences technology and been a part of the transformation experiment, but this testing was not performed because of limited resources. In addition, the decisions regarding which isolates to select for transformation experiments and sequencing by using Pacific Biosciences technology were made before WGS was complete. In retrospect, it would have been better to select blaOXA-181 isolates that were hypothesized to be on a plasmid for the transformation experiment; instead, chromosomal blaOXA-181 isolates were selected. Thus, the blaOXA-181 gene loci for the isolates in this study are inconclusive.
In summary, the continued increase of CRE in the United States is a major problem, and the increasing prevalence of OXA-48–like carbapenemases is also concerning. We found Enterobacteriaceae in the United States with blaOXA-48−like genes on similar mobile genetic elements to those described elsewhere and that displayed relatively resistant AST profiles. The first step in continued detection of CRE producing these and other carbapenemases is identifying all carbapenem resistance among Enterobacteriaceae, including resistance to ertapenem. Future prospective investigations are needed to determine the true prevalence of OXA-48–like carbapenemases in the United States.
Dr. Lutgring is an assistant professor of medicine at Emory University School of Medicine, Atlanta, GA. His primary research interest is the molecular mechanisms of antimicrobial resistance in gram-negative bacteria.
Acknowledgment
This study was supported by the Advanced Molecular Detection Program at CDC.
References
- Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53:60–7. DOIPubMed
- Centers for Disease Control and Prevention (CDC). Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–70.PubMed
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–106. DOIPubMed
- Walther-Rasmussen J, Høiby N. Class A carbapenemases. J Antimicrob Chemother. 2007;60:470–82. DOIPubMed
- Guh AY, Bulens SN, Mu Y, Jacob JT, Reno J, Scott J, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012–2013. JAMA. 2015;314:1479–87. DOIPubMed
- Lascols C, Peirano G, Hackel M, Laupland KB, Pitout JD. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: first report of OXA-48-like enzymes in North America. Antimicrob Agents Chemother. 2013;57:130–6. DOIPubMed
- Mathers AJ, Hazen KC, Carroll J, Yeh AJ, Cox HL, Bonomo RA, et al. First clinical cases of OXA-48-producing carbapenem-resistant Klebsiella pneumoniae in the United States: the “menace” arrives in the new world. J Clin Microbiol. 2013;51:680–3. DOIPubMed
- Doi Y, O’Hara JA, Lando JF, Querry AM, Townsend BM, Pasculle AW, et al. Co-production of NDM-1 and OXA-232 by Klebsiella pneumoniae. Emerg Infect Dis. 2014;20:163–5. DOIPubMed
- Yang S, Hemarajata P, Hindler J, Li F, Adisetiyo H, Aldrovandi G, et al. Evolution and transmission of carbapenem-resistant Klebsiella pneumoniae expressing the blaOXA-232 gene during an institutional outbreak associated with endoscopic retrograde cholangiopancreatography. Clin Infect Dis. 2017;64:894–901. DOIPubMed
- Rojas LJ, Hujer AM, Rudin SD, Wright MS, Domitrovic TN, Marshall SH, et al. NDM-5 and OXA-181 beta-lactamases, a significant threat continues to spread in the Americas. Antimicrob Agents Chemother. 2017;61:e00454–17. DOIPubMed
- Poirel L, Héritier C, Tolün V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48:15–22. DOIPubMed
- Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 2012;67:1597–606. DOIPubMed
- Oteo J, Hernández JM, Espasa M, Fleites A, Sáez D, Bautista V, et al. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain.J Antimicrob Chemother. 2013;68:317–21. DOIPubMed
- Sampaio JL, Ribeiro VB, Campos JC, Rozales FP, Magagnin CM, Falci DR, et al. Detection of OXA-370, an OXA-48-related class D β-lactamase, in Enterobacter hormaechei from Brazil.Antimicrob Agents Chemother. 2014;58:3566–7. DOIPubMed
- Meunier D, Vickers A, Pike R, Hill RL, Woodford N, Hopkins KL. Evaluation of the K-SeT R.E.S.I.S.T. immunochromatographic assay for the rapid detection of KPC and OXA-48-like carbapenemases. J Antimicrob Chemother. 2016;71:2357–9. DOIPubMed
- Pasteran F, Denorme L, Ote I, Gomez S, De Belder D, Glupczynski Y, et al. Rapid identification of OXA-48 and OXA-163 subfamilies in carbapenem-resistant gram-negative bacilli with a novel immunochromatographic lateral flow assay. J Clin Microbiol. 2016;54:2832–6. DOIPubMed
- Dortet L, Naas T. Noncarbapenemase OXA-48 variants (OXA-163 and OXA-405) falsely detected as carbapenemases by the β Carba test. J Clin Microbiol. 2017;55:654–5. DOIPubMed
- Gomez S, Pasteran F, Faccone D, Bettiol M, Veliz O, De Belder D, et al. Intrapatient emergence of OXA-247: a novel carbapenemase found in a patient previously infected with OXA-163-producing Klebsiella pneumoniae. Clin Microbiol Infect. 2013;19:E233–5. DOIPubMed
- Lyman M, Walters M, Lonsway D, Rasheed K, Limbago B, Kallen A. Notes from the field: carbapenem-resistant Enterobacteriaceae producing OXA-48-like carbapenemases—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2015;64:1315–6. DOIPubMed
- Lascols C, Bonaparte S, Lonsway D, Johnson K, Robinson G, Rasheed K, et al. Snapshot of beta-lactam resistance in Enterobacteriaceae in the United States. Poster 1051. In: Abstracts of the 25th European Congress of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark, April 25–28, 2015. Abstract 1051 [cited 2018 Jan 26] https://www.escmid.org/dates_events/calendar/calendar_event/cal/2015/04/25/event/tx_cal_phpicalendar/25th_European_Congress_of_Clinical_Microbriology_and_Infectious_Diseases_ECCMID_2015/?tx_cal_controller%5Blastview%5D=view-search_event%7Cpage_id-130&cHash=fc21e6af2b4d39892216b2e8d30f0936
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. 10th ed. (M07-A10). Wayne (PA): The Institute; 2015.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty-seventh informational supplement. (M100-S27). Wayne (PA): The Institute; 2017.
- Conrad S, Oethinger M, Kaifel K, Klotz G, Marre R, Kern WV. gyrA mutations in high-level fluoroquinolone-resistant clinical isolates of Escherichia coli. J Antimicrob Chemother. 1996;38:443–55. DOIPubMed
- Kitchel B, Zhu W, Travis T, Limbago BM, Rasheed JK. Detection and evaluation of OXA-48 like carbapenemases by real-time PCR. Poster D-1139. In: Abstracts of the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, Colorado, September 10–13, 2013 cited 2018 Jan 26]. https://www.medscape.com/viewcollection/32893
- Cox MP, Peterson DA, Biggs PJ, Solexa QA. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics. 2010;11:485. DOIPubMed
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–77. DOIPubMed
- Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–95. DOIPubMed
- Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–9. DOIPubMed
- Inouye M, Dashnow H, Raven LA, Schultz MB, Pope BJ, Tomita T, et al. SRST2: Rapid genomic surveillance for public health and hospital microbiology labs. Genome Med. 2014;6:90. DOIPubMed
- de Man TJ, Limbago BM. SSTAR: a stand-alone easy-to-use antimicrobial resistance gene predictor. mSphere. 2016;1:pii: e00050-25. PMID: 27303709.DOI
- Gupta SK, Padmanabhan BR, Diene SM, Lopez-Rojas R, Kempf M, Landraud L, et al. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes.Antimicrob Agents Chemother. 2014;58:212–20. DOIPubMed
- Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–4. DOIPubMed
- Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing.Antimicrob Agents Chemother. 2014;58:3895–903. DOIPubMed
- Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34:D32–6. DOIPubMed
- Potron A, Nordmann P, Rondinaud E, Jaureguy F, Poirel L. A mosaic transposon encoding OXA-48 and CTX-M-15: towards pan-resistance. J Antimicrob Chemother. 2013;68:476–7. DOIPubMed
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. DOIPubMed
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3. DOIPubMed
- Durfee T, Nelson R, Baldwin S, Plunkett G III, Burland V, Mau B, et al. The complete genome sequence of Escherichia coli DH10B: insights into the biology of a laboratory workhorse. J Bacteriol. 2008;190:2597–606. DOIPubMed
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9. DOIPubMed
- Karim A, Poirel L, Nagarajan S, Nordmann P. Plasmid-mediated extended-spectrum beta-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol Lett. 2001;201:237–41.PubMed
- McGann P, Snesrud E, Ong AC, Appalla L, Koren M, Kwak YI, et al. War wound treatment complications due to transfer of an IncN plasmid harboring bla(OXA-181) from Morganella morganii to CTX-M-27-producing sequence type 131 Escherichia coli. Antimicrob Agents Chemother. 2015;59:3556–62. DOIPubMed
- Potron A, Nordmann P, Lafeuille E, Al Maskari Z, Al Rashdi F, Poirel L. Characterization of OXA-181, a carbapenem-hydrolyzing class D beta-lactamase from Klebsiella pneumoniae.Antimicrob Agents Chemother. 2011;55:4896–9. DOIPubMed
- Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, Camiade S, et al. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int J Antimicrob Agents. 2013;41:325–9. DOIPubMed
- Chen L, Al Laham N, Chavda KD, Mediavilla JR, Jacobs MR, Bonomo RA, et al. First report of an OXA-48-producing multidrug-resistant Proteus mirabilis strain from Gaza, Palestine.Antimicrob Agents Chemother. 2015;59:4305–7. DOIPubMed
- Papagiannitsis CC, Študentová V, Izdebski R, Oikonomou O, Pfeifer Y, Petinaki E, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry meropenem hydrolysis assay with NH4HCO3, a reliable tool for direct detection of carbapenemase activity. J Clin Microbiol. 2015;53:1731–5. DOIPubMed
- Tijet N, Boyd D, Patel SN, Mulvey MR, Melano RG. Evaluation of the Carba NP test for rapid detection of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2013;57:4578–80. DOIPubMed
- Chea N, Bulens SN, Kongphet-Tran T, Lynfield R, Shaw KM, Vagnone PS, et al. Improved phenotype-based definition for identifying carbapenemase producers among carbapenem-resistant Enterobacteriaceae. Emerg Infect Dis. 2015;21:1611–6. DOIPubMed
- Carrër A, Poirel L, Eraksoy H, Cagatay AA, Badur S, Nordmann P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob Agents Chemother. 2008;52:2950–4. DOIPubMed
- Poirel L, Bonnin RA, Nordmann P. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother. 2012;56:559–62. DOIPubMed
- Villa L, Carattoli A, Nordmann P, Carta C, Poirel L. Complete sequence of the IncT-type plasmid pT-OXA-181 carrying the blaOXA-181 carbapenemase gene from Citrobacter freundii.Antimicrob Agents Chemother. 2013;57:1965–7. DOIPubMed


































No hay comentarios:
Publicar un comentario