
Timing and Sequence Critical for Immunotherapy Combination
October 3, 2017, by NCI Staff
A new study in mice suggests that for treatment that combines two immunotherapy drugs, the timing and sequence of the drugs’ administration are critical to the treatment’s efficacy and safety.
The study investigators found that treating mice with two different immunotherapy agents at the same time had less of an effect on tumor growth compared with one of the agents alone. But staggering the timing of when the two therapieswere given substantially slowed tumor growth and extended survival in the mice. However, this effect was not observed when the therapies were given in the reverse order.
“Studying these mice has given us some insight about how important the schedule [of combination treatment] is,” said the study’s senior investigator Bernard A. Fox, Ph.D., of the Earle A. Chiles Research Institute, Robert W. Franz Cancer Center.
The findings from the study, which was funded in part by NCI and appeared August 28 in Clinical Cancer Research, reinforce that “we need to invest in studying patients more closely, and do really well-controlled clinical trials of immunotherapy combinations,” Dr. Fox added. “I think that’s how we’re going to make greater progress.”
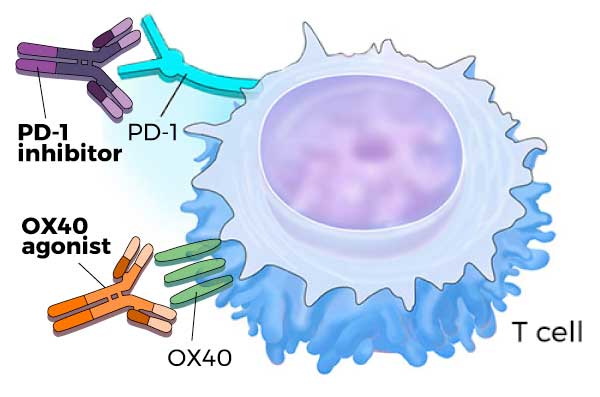
Joining Forces: PD-1 Inhibitors and OX40 Agonists
A T cell is a type of immune cell that has the ability to attack and kill infected or abnormal cells, including cancer cells. This killing ability is carefully controlled by several molecules on the surface of the T cell. For example, PD-1 limits a T cell’s killing ability, while OX40 enhances it.
Targeted therapies that block PD-1 on T cells or its binding partner, PD-L1, on tumor cells lead to increased T-cell activity and a potentially greater antitumor immune response. Several of these so-called immune checkpoint inhibitors have been approved by the Food and Drug Administration to treat certain cancers, including lung cancer and melanoma.
However, PD-1 and PD-L1 inhibitors have demonstrated limited efficacy for patients with many other types of cancer, including breast cancer.
“What we’re struggling with now is figuring out how to take the really deep, durable, and rapid responses we’re seeing with some immunotherapies and broaden the group of patients who have these phenomenal responses,” said James Gulley, M.D., Ph.D., head of the immunotherapy section of NCI’s Center for Cancer Research, who was not involved in the study.
For patients who do not respond to immunotherapy, one problem appears to be that their tumors counteract the immune response induced by these drugs, Dr. Gulley explained. “With a single immunotherapy, the tumor can find a way to shut down the antitumor immune response,” he said.
Dr. Fox and his team wanted to see if, for tumors that do not respond to PD-1 inhibitors, combining the treatment with another type of immunotherapy might be able to overcome these counter measures and generate a more effective antitumor immune response.
They turned to a new class of immunotherapy drugs known as OX40 agonists—drugs that bind to and activate OX40, potently ramping up T-cell activity. Several OX40 agonists are currently being evaluated in clinical trials as potential cancer treatments.
To test their hypothesis, the research team used a mouse model of breast cancer that closely resembles how the disease behaves in humans. After tumors formed in the mice, the researchers left them untreated or treated them with a PD-1 inhibitor, an OX40 agonist, or both immunotherapy agents at the same time.
Compared with untreated mice, PD-1 inhibitor treatment alone had no impact on tumor growth or survival, whereas OX40 agonist treatment alone slowed tumor growth and improved survival, they found.
The combination treatment, however, had less of an effect on tumor growth than the OX40 agonist alone. In addition, the survival benefit observed with OX40 agonist treatment alone was diminished with the combination treatment.
“We were really surprised to see that the addition of those two agents together was significantly less effective” than the OX40 agonist alone, Dr. Fox said.
The researchers also found that mice treated with the combination had very high levels of certain cytokines and exhibited symptoms of cytokine release syndrome (CRS), a side effect of some immunotherapies. CRS is “typically the result of hyperactive T cells that generate massive amounts of cytokines, resulting in symptoms such as low blood pressure, fever, nausea, and rash,” said Dr. Gulley. In some cases, CRS can become severe, resulting in organ failure and even death.
In addition, the combination treatment eventually caused T cells in the mouse tumors to shut down—an effect known as T-cell exhaustion.
The team wondered if sequential treatment with the two drugs might work better to slow tumor growth in the mice. They reasoned that treatment with the OX40 agonist first might provide an initial boost in antitumor T-cell activity, and subsequent treatment with the PD-1 inhibitor might extend it.
Their hunch was accurate: Treatment with the OX40 agonist followed by treatment with the PD-1 inhibitor 2 days later delayed tumor growth and extended the mice’s survival more than treatment with the OX40 agonist alone.
In fact, the sequential combination treatment led to complete tumor regression in 30% of the mice and nearly doubled the group’s survival time compared with concurrent combination treatment. And the sequential treatment did not lead to CRS-like symptoms or T-cell exhaustion.
However, reversing the treatment sequence—treating mice with the PD-1 inhibitor followed by the OX40 agonist—did not slow tumor growth.
Separating the administration of the two drugs “had a profound effect on the biological effects of the drugs,” said Dr. Fox. “The most striking thing was the fact that we could get such long-term survival and apparent cure in some mice if we sequence the therapies,” he added. This kind of response has not been observed in other preclinical studies using the same mouse model, he noted.
Considerations for Immunotherapy Clinical Trials
Dr. Gulley noted that although mouse models are not the perfect surrogate for human biology, they can give good insight into the potential effects of combination treatments in humans.
“This study prompts us to take a step back and say, let’s not just haphazardly combine immunotherapies. Let’s understand the biology of what we’re doing before we do clinical trials,” he said.
Dr. Fox believes combination immunotherapies might be even more effective if a precision medicine approach is incorporated. By examining molecular biomarkers in an individual patient’s tumor, he explained, doctors may better predict which immunotherapies will generate the most effective antitumor immune response in the patient.
“We’re not there yet, but the idea is that we’ll be able to tailor therapy to each individual,” he said.
For example, PD-1 inhibitors and OX40 agonists both strengthen a weak antitumor immune response, so they are best suited for patients with an existing immune response. But patients who have no antitumor immune response at all—which is probably the majority, said Dr. Fox—may require another type of immunotherapy to jump-start their immune systems.
Scientists are testing PD-1 inhibitors and OX40 agonists with other immunotherapies that can trigger an initial antitumor immune response, Dr. Gulley noted.
For example, Dr. Fox and his colleagues are exploring a combination treatment including a cancer vaccine and an OX40 agonist in patients with advanced cancer. In preclinical studies the combination treatment significantly increased antitumor T-cell activity, and the cancer vaccine was recently tested in an NCI-funded phase II trial as adjuvant therapyfor patients at high risk of cancer recurrence. They plan to test the vaccine in combination with an OX40 agonist and a PD-1 inhibitor in a subsequent trial.





















.png)
.png)











No hay comentarios:
Publicar un comentario