
 |
Developmental Neurotoxicity Associated With Pediatric General Anesthesia
NCTR scientists have published a review articlethat focuses on preclinical studies of general anesthesia-induced effects on brain and behavioral development. The authors address multiple anesthetic agents and their effects on various aspects of neurotoxicity and functional outcomes, potential protective compounds, and potential areas of future research.
This review was published in a special issue of Neurotoxicity and Teratology devoted to “Developmental Neurotoxicity Associated with Pediatric General Anesthesia: Preclinical Findings,” also co-edited by an NCTR scientist.
For more information, please contact Merle Paule, Ph.D., Division Director, Division of Neurotoxicology.
Save the Date:
2017 Global Summit for Regulatory Science
- THEME: Emerging Technologies for Drug and Food Safety
- DATE: September 18-20, 2017
- LOCATION: Brasilia, Brazil
 |
FDA/NCTR will be co-hosting the 2017 Global Summit for Regulatory Science with the Brazilian Health Regulatory Agency — ANVISA — to be held in Brasilia, BRAZIL on September 18-20, 2017. The Global Summit on Regulatory Science (GSRS) is an international conference for discussion of innovative technologies and partnerships to enhance translation of basic science into regulatory applications within the global context. To engage the global community to address regulatory-science research and training needs, GSRS is held in different countries on an annual basis.
The conference provides an opportunity for scientists from government, industry, and academic-research communities to objectively assess the utility of emerging technologies for addressing regulatory-research questions and to discuss the best way to translate these technologies into real-world applications. It provides a platform where regulators, policy makers, and bench scientists from various countries can exchange views on how to develop, apply, and implement innovative methodologies into regulatory assessments in their respective countries, as well as harmonizing strategy via global collaboration.
The theme for this year’s GSRS is “Emerging Technologies for Drug and Food Safety” and in addition to panel discussions and poster sessions, the agenda will include the following scientific topics:
- Global Regulatory Landscape
- Emerging Fields and Methodologies
- Drug and Food Safety
- Towards Acceptance of New Approaches to Safety Assessment: Standards and Reproducibility
- Science-Based Regulatory Practice.
There is no registration fee; however, registration is required to attend the conference.
For recurring updates, visit www.fda.gov/globalsummit
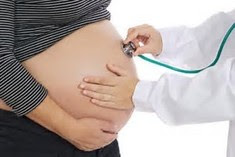 |
Risk Evaluation of Perchlorate Exposure in Pregnant Women Using Probabilistic Biologically Based Dose-Response (BBDR) Modeling
NCTR scientists developed a probabilistic biologically based dose-response (BBDR) model to predict thyroid effects (as indicated by reductions in maternal serum free thyroxin, fT4) of perchlorate exposure in late-gestation pregnant women. The model predicted a decrease in fT4 levels from perchlorate exposure only when drinking water exposures were combined with food for a total daily intake of 0.45 to 0.50 µg/kg/day of perchlorate. Based on the model, drinking water concentrations of perchlorate less than 7.6-9.2 µg/L were not found to cause a statistically significant reduction in maternal fT4 levels.
The U.S. population is ubiquitously exposed to perchlorate through food and drinking water. Perchlorate inhibits the uptake of iodide in the thyroid, which decreases thyroid hormone production. Pregnant women are particularly sensitive since decreases in maternal thyroid hormones have been associated with fetal neurodevelopmental defects.
This study demonstrated the potential application of a probabilistic BBDR model for perchlorate risk assessment in a sensitive life-stage at a population level. This work was supported in part by the FDA Office of Women’s Health. A manuscript describing the study is available online at Toxicology and Applied Pharmacology.
For more information, please contact Annie Lumen, Ph.D., Division of Biochemical Toxicology or Nysia George, Ph.D., Division of Bioinformatics and Biostatistics.
Nanotechnology Training
at NCTR
The NCTR/ORA Nanotechnology Core Facility — located on the Jefferson Laboratories campus in Arkansas — conducted a “Nanotechnology Hands-on Training” course from June 27-29, 2017. In attendance were 12 FDA reviewers and research staff from different FDA product centers and offices. This annual training was sponsored by the FDA Nanotechnology Task Force/Office of Chief Scientist with instructors from across FDA from NCTR, Office of Regulatory Affairs, Center for Drug Evaluation and Research, and Center for Devices and Radiological Health. The lecture topics included:
- basic tools and instrumentation used for nanomaterial characterization (Transmission and Scanning Electron Microscopy, Atomic Force Microscopy, Dynamic Light Scattering and Asymmetric, and Centrifugal Flow Field Flow Fractionation)
- regulatory considerations for generic and new drug products and devices containing nanomaterial
- nanomaterial characterization
- evaluation of data
- nanomaterial and product specific issues to be considered when reviewing submissions.
For additional information, please contact Anil Patri, Ph.D., Director, NCTR/ORA Nanotechnology Core Facility.





















.png)












No hay comentarios:
Publicar un comentario