A new DRUG TRIALS SNAPSHOT is now available.

XCOPRI is a drug for the treatment of specific type of seizures called partial-onset seizures in adult patients.
Partial-onset seizures occur when clusters of nerve cells (neurons) undergo uncontrolled activation in a limited area of the brain.
XCOPRI is a tablet drug that is taken once daily by mouth. An initial low dose may be increased every two weeks according to a special schedule.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots: XCOPRI
XCOPRI (cenobamate)
ex koe' pree
SK Life Science, Inc.
Approval date: November 21, 2019
ex koe' pree
SK Life Science, Inc.
Approval date: November 21, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
XCOPRI is a drug for the treatment of specific type of seizures called partial-onset seizures in adult patients.
Partial-onset seizures occur when clusters of nerve cells (neurons) undergo uncontrolled activation in a limited area of the brain.
How is this drug used?
XCOPRI is a tablet drug that is taken once daily by mouth. An initial low dose may be increased every two weeks according to a special schedule.
What are the benefits of this drug?
Patients taking XCOPRI had less frequent seizures than patients taking placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: XCOPRI worked similarly in men and women.
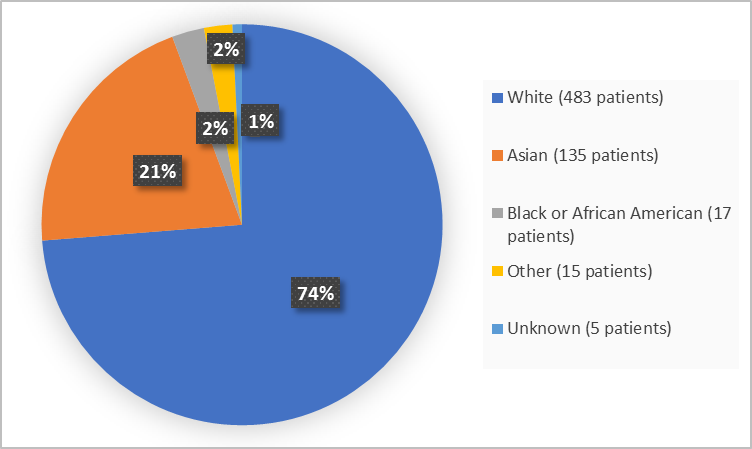
- Race: The majority of patients in the clinical trial were White. Differences in how well XCOPRI worked among different races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in how well XCOPRI worked between patients below and above 65 years of age could not be determined.
What are the possible side effects?
XCOPRI may cause serious side effects including life-threatening allergic reactions (called Drug Reaction with Eosinophilia and Systemic Symptoms or DRESS), thoughts about suicide or dying, heart rhythm problems (because of a shortened QT interval), and drowsiness which may impair driving ability.
The most common side effects of XCOPRI are drowsiness, dizziness, feeling tired, double vision, and headache.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women
- Race: The occurrence of side effects was similar among tested race groups.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in side effects between patients below and above 65 years of age could not be determined.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved XCOPRI based primarily on evidence from two clinical trials (Trial 1/NCT01866111 and Trial2/NCT01397968) of 655 patients with partial-onset seizures.
The trials were conducted at 147 sites in the USA, Europe, Asia, Australia, Israel, and South Africa.
The figure below summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical Trial Data
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
Clinical Trial Data
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical Trial Data
How were the trials designed?
The benefit and side effects of XCOPRI were evaluated in two clinical trials of patients with partial-onset seizures who were not adequately controlled on their current therapy. Both trials had an 8-week period to assess baseline seizure frequency while patients were taking their usual treatments for seizures. After 8 weeks and in addition to their usual treatments for seizures, patients received either XCOPRI tablets at various doses or placebo tablets for 12 weeks. Neither the patients nor the health care providers knew which new treatment was being given until after the trial was completed.
The benefit of XCOPRI was evaluated by measuring the difference from baseline in number of seizures per 28 days of treatment and comparing it to the placebo.
Patients from both trials who completed the treatment with either XCOPRI or placebo were eligible to continue taking XCOPRI in order to collect long-term safety data.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.




































No hay comentarios:
Publicar un comentario