A new DRUG TRIALS SNAPSHOT is now available.

VYONDYS 53 is a drug for the treatment of a particular type of Duchenne muscular dystrophy (DMD). It is to be used only in patients who have a specific mutation of the dystrophin gene.
DMD is a rare disease that primarily affects boys. It is caused by low levels of a muscle protein called dystrophin. The lack of dystrophin causes progressive muscle weakness and premature death.
VYONDYS 53 is given by a health care professional once every week directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV infusion.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov
Drug Trials Snapshots: VYONDYS 53
VYONDYS 53 (golodirsen)
vy-ON-dys
Serepta Therapeutics Inc.
Approval date: December 12, 2019
vy-ON-dys
Serepta Therapeutics Inc.
Approval date: December 12, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VYONDYS 53 is a drug for the treatment of a particular type of Duchenne muscular dystrophy (DMD). It is to be used only in patients who have a specific mutation of the dystrophin gene.
DMD is a rare disease that primarily affects boys. It is caused by low levels of a muscle protein called dystrophin. The lack of dystrophin causes progressive muscle weakness and premature death.
How is this drug used?
VYONDYS 53 is given by a health care professional once every week directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV infusion.
What are the benefits of this drug?
VYONDYS 53 increased levels of dystrophin in the muscles of most patients. It is believed that this increase may predict clinical benefit in patients.
VYONDYS 53 was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
More trials are ongoing to assess whether there is a clinical benefit of VYONDYS 53.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The trial that looked at the benefit of VYONDYS 53 consisted of boys only who were of similar age and predominantly White. It was not possible to determine if there were any differences in how well the drug worked in sex, race and age subgroups.
What are the possible side effects?
VYONDYS 53 may cause serious allergic reactions. Additionally, kidney damage was observed in animal studies.
The most common side effects of VYONDYS 53 are headache, fever, falling, abdominal pain, common cold, cough, vomiting, and nausea.
Were there any differences in side effects among sex, race and age?
The trials that looked at the side effects of VYONDYS 53 consisted of boys only, who were of similar age and predominantly White. It was not possible to determine if there were any differences in side effects in sex, race and age subgroups.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
There were two clinical trials that enrolled patients with DMD (Trial 1/NCT02310906 and Trial 2/NCT0250381). The trials were conducted in the United States and Europe.
Demographics of the combined populations from both trials that provided data for evaluation of side effects (called safety population) are presented below and in Table 4 under MORE INFO.
Only one part of Trial 1 provided data for evaluation of VYONDYS 53 benefits. Demographics of that population (called efficacy population) are presented in Table 5 under MORE INFO.
The figure below summarizes patients in combined Trials 1 and 2, by sex.
Figure 1. Baseline Demographics by Sex (safety population)
Clinical Trial Data
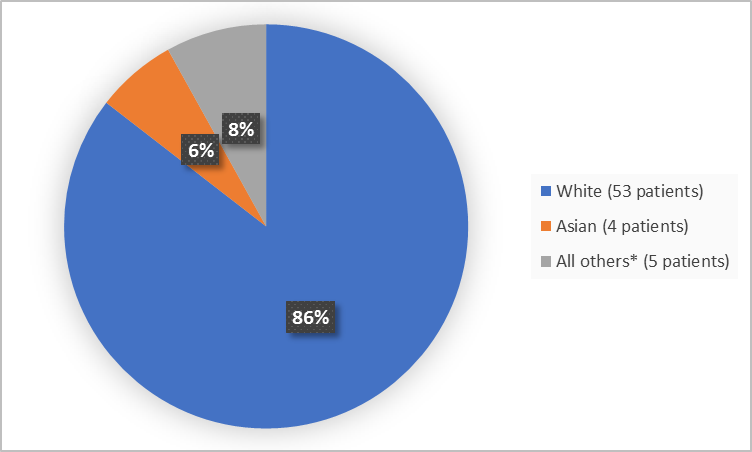
Figure 2 summarizes the percentage of patients by race in Trials 1 and 2.
Figure 2. Baseline Demographics by Race (safety population)
*Includes Black or African American, American Indian or Alaska Native and Other
Clinical Trial Data
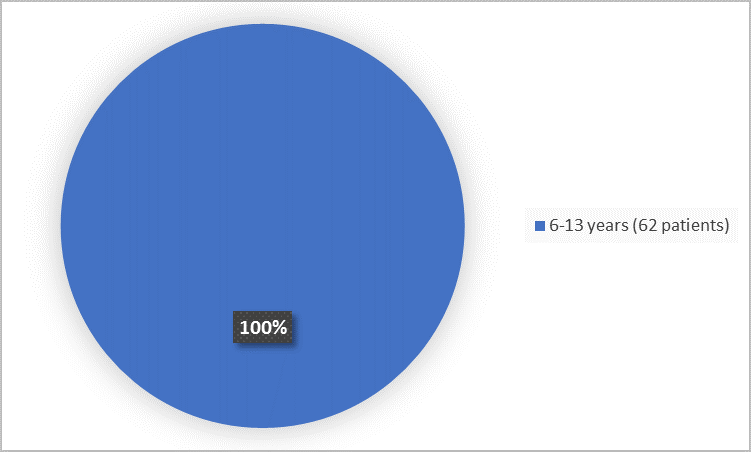
Figure 3 summarizes the percentage of patients by age in Trials 1 and 2.
Figure 3. Baseline Demographics by Age (safety population)
Clinical Trial Data
How were the trials designed?
There were two trials that evaluated VYONDYS 53 for DMD. Data from Trial 1 was used to evaluate the benefit and side effects of VYONDYS 53 and data from Trial 2 to evaluate side effects only. All patients were on a stable dose of corticosteroids for at least 6 months before entering the trials.
In Trial 1, patients were randomly assigned to receive either VYONDYS 53 or placebo once a week. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed. The benefit was evaluated by measuring the level of dystrophin in muscle biopsies before and after 48 weeks of treatment.
In Trial 2, patients received VYONDYS 53 or placebo once a week for up to 96 weeks. Neither the patients nor the health care providers knew which treatment was being given. Collected data was used for side effects assessment.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.



































No hay comentarios:
Publicar un comentario