A new DRUG TRIALS SNAPSHOT is now available.

ADAKVEO is used to reduce the frequency of certain crises episodes (called vaso-oclusive crises) in patients 16 years of age and older who have sickle cell disease.
Sickle cell is an inherited blood disorder in which the red blood cells are abnormally shaped (in a crescent or "sickle" shape). Vaso-oclusive crisis (VOC) is a common and painful complication of sickle cell disease that occurs when blood circulation is obstructed by sickled red blood cells leading to severe pain and organ damage.
ADAKVEO is given by a healthcare provider directly into the vein (intravenous infusion) over 30 minutes. First two infusions are given 2 weeks apart followed by an infusion every 4 weeks thereafter.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots ADAKVEO
ADAKVEO (crizanlizumab-tmca)
ah dak vee oh
Novartis Pharmaceuticals Corporation
Approval date: November 15, 2019
ah dak vee oh
Novartis Pharmaceuticals Corporation
Approval date: November 15, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ADAKVEO is used to reduce the frequency of certain crises episodes (called vaso-oclusive crises) in patients 16 years of age and older who have sickle cell disease.
Sickle cell is an inherited blood disorder in which the red blood cells are abnormally shaped (in a crescent or "sickle" shape). Vaso-oclusive crisis (VOC) is a common and painful complication of sickle cell disease that occurs when blood circulation is obstructed by sickled red blood cells leading to severe pain and organ damage.
How is this drug used?
ADAKVEO is given by a healthcare provider directly into the vein (intravenous infusion) over 30 minutes. First two infusions are given 2 weeks apart followed by an infusion every 4 weeks thereafter.
What are the benefits of this drug?
Patients treated with ADAKVEO experienced fewer health care visits for VOC per year (about 1.63 visits), compared to patients who received a placebo (about 2.98 visits).
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
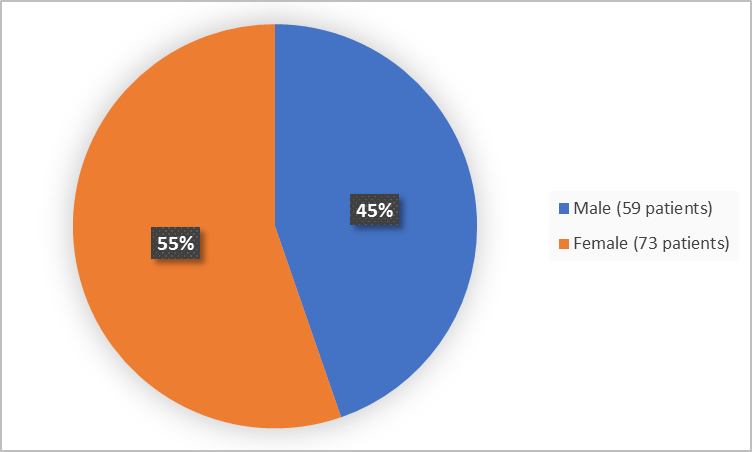
- Sex: ADAKVEO worked similarly in males and females.
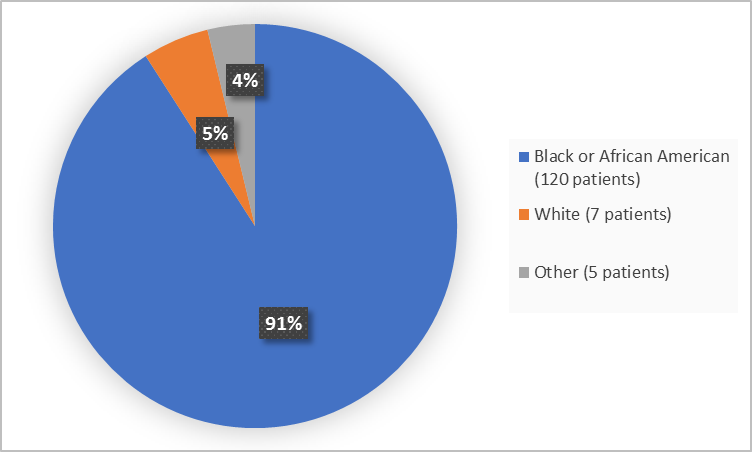
- Race: The majority of patients were Black or African Americans. The number of patients in other races were limited; therefore, differences in how ADAKVEO worked among races could not be determined.
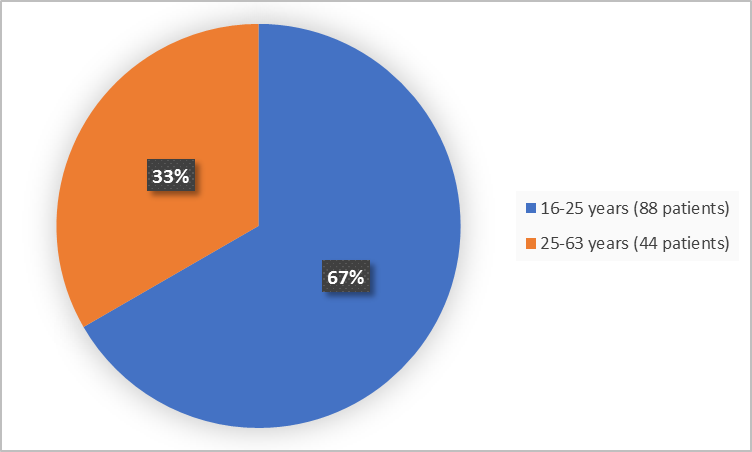
- Age: The majority of patients were adults between 18-63 years of age. There were not enough patients older than 65 years to determine whether there was any difference in how ADAKVEO worked between older and younger patients.
What are the possible side effects?
ADAKVEO may cause serious infusion-related reactions and platelet clumping.
Most common side effects of ADAKVEO include nausea, joint pain, back pain and fever.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar between males and females.
- Race: The majority of patients were Black or African Americans. The number of patients in other races were limited; therefore, differences in how ADAKVEO worked among races could not be determined.
- Age: The majority of patients were adults between 18-63 years of age. There were not enough patients older than 65 years to determine whether there was any difference in side effects between older and younger patients.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved ADAKVEO based on evidence from one clinical trial (Trial 1/NCT01895361) of 132 patients with SS diseases who had a history of VOC. The trial was conducted at 60 sites in the United States, Brazil and Jamaica.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Review
Figure 3 summarizes the percentage of patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
There was one trial that evaluated the benefit and side effects of ADAKVEO in patients with SS disease. Patients had to have a history of 2-10 VOCs in the previous 12 months. Patients received at random either ADAKVEO or placebo infusions. The first two infusions were given 2 weeks apart followed by an infusion every 4 weeks. Trials lasted 52 weeks. Nether the patients not the healthcare providers knew which treatment had been given until the end of the trial.
The benefit of ADAKVEO was assessed by counting the number of VOCs healthcare visits that required pain treatments over a 1-year period.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.


































No hay comentarios:
Publicar un comentario