
Volume 24, Number 3—March 2018
Dispatch
Cache Valley Virus in Aedes japonicus japonicus Mosquitoes, Appalachian Region, United States
On This Page
Fan Yang1, Kevin Chan, Paul E. Marek, Philip M. Armstrong, Pengcheng Liu, Jacob E. Bova, Joshua N. Bernick, Benjamin E. McMillan, Benjamin G. Weidlich, and Sally L. Paulson
Abstract
We detected Cache Valley virus in Aedes japonicus, a widely distributed invasive mosquito species, in an Appalachian forest in the United States. The forest contained abundant white-tailed deer, a major host of the mosquito and virus. Vector competence trials indicated that Ae. j. japonicusmosquitoes can transmit this virus in this region.
Cache Valley virus (CVV; family Bunyaviridae, genus Orthobunyavirus) is widespread throughout North and Central America and infects many species of domestic ungulates (sheep and cattle), but white-tailed deer are a likely reservoir (1). Although this virus has been isolated from >30 mosquito species in several genera, the principal vectors remain unknown (2,3). However, on the basis of field isolations and laboratory transmission studies, Anopheles quadrimaculatus and An. punctipennis mosquitoes probably play major roles in its transmission cycle (1,4).
CVV infection is common in sheep and causes spontaneous abortion, stillbirth, and congenital defects (5). The virus is neuroinvasive in humans, and there have been 3 confirmed cases and 1 death in the United States (2,6). Medical laboratories rarely test for CVV, which underestimates its true incidence and effect on human health, but serologic studies have reported high human infection rates (up to 18%) in virus-endemic areas (7). We report detection of CVV in the invasive mosquito Aedes japonicus japonicus in Blacksburg, Virginia, USA, and demonstrate that this species is a competent vector of the virus.
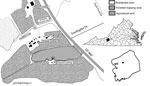
Figure 1. Study site used for detection of Cache Valley virus in Aedes japonicus japonicus mosquitoes, Blacksburg, Virginia, USA, 2015. Insets show location of Blacksburg in Montgomery County (black box) and the county...
We collected adult mosquitoes during June 1–August 21, 2015, by using gravid traps in a forested area (area 196,115 m2) (Figure 1). We collected 1,197 Ae. triseriatus and 690 Ae. j. japonicus adult female mosquitoes; identified them to species on the basis of morphology; and pooled them by species, trap number, and date (Table 1). Pools (626 of Ae. triseriatus and 442 of Ae. j. japonicus) consisting of 1–50 mosquitoes were stored at −80°C. We screened samples on Vero cells for cytopathic effect and confirmed the presence of virus in positive samples by using a plaque assay (8).
We amplified virus isolates on Vero cells to a titer of 105 PFU/mL and extracted virus RNA from infected cell supernatants by using the QIAamp Viral RNA Mini Kit (QIAGEN, Valencia, CA, USA). We used reverse transcription PCR and Bunyaviridae-specific universal primers BCS82C and BCS332V to produce a 251-bp amplicon of the small RNA segment, which was then sequenced (9). Sequencing was performed by Eton Bioscience, Inc. (San Diego, CA, USA). A BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) query indicated that the isolates were CVV.
We amplified large RNA segments by using reverse transcription PCR and CVV-specific primers CVV_L (5′-AGTCAGCCAAAACAGCCACT-3′) and CVV_R (5′-TACAAATCTAGGGGGCATGG-3′) and amplified medium RNA segments by using primers M14C and M4510R (9,10). Resulting amplicons were sequenced and identified as CVV by performing a BLAST query. Medium RNA segments encoding the Gc protein were amplified by using the primer pair CVV_M_L (5′-CTGTCACGGTGCTAGTAGGAAAGATGTG-3′) and CVV_M_R (5′-AGTAGTGTGCTACCGGTATCAAAAACAGC-3′) and then sequenced.
We detected CVV in 2 Ae. j. japonicus female mosquitoes collected from different traps on August 7 (Table 1). For the week of August 4–11, we calculated the CVV minimum infection rate to be 11.5/1,000 mosquitoes (173 mosquitoes tested individually). This late-season occurrence of CVV is consistent with results of a study in Connecticut, USA (4). Although Ae. triseriatus mosquitoes were more abundant than Ae. j. japonicus mosquitoes and have a similar biology as the invasive mosquito, we did not detect CVV in Ae. triseriatus mosquitoes.
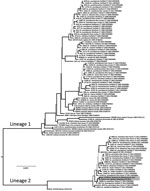
Figure 2. Phylogeny of Cache Valley virus (CVV) isolates in mosquitoes collected in Blacksburg, Virginia, USA (GenBank accession nos. KX583998 and KX583999), and reference isolates. The tree was inferred from the medium RNA...
We used the medium RNA segment to infer phylogeny with CVV isolates reported by Armstrong et al. (10). We translated RNA sequences to amino acid sequences and aligned them visually, which was trivial because of invariant length and lack of insertion−deletion events. The alignment was composed of 1,803 bp from 100 isolates. We partitioned sequences by codon position and evaluated alternative models of nucleotide site substitution. We inferred phylogenetic trees by using a Markov chain Monte Carlo method in MrBayes 3.2.5 with a simultaneous estimation of topology, branch lengths, and other parameters (11). Stabilization of 4 concurrent chains occurred at 1 million generations, and the first 250,000 trees were discarded as a burn-in. We averaged branch lengths and other parameters and constructed a consensus tree (Figure 2) from the posterior distribution that contained support values for each clade in posterior probabilities.
CVV isolates were grouped into 2 clades or lineages (Figure 2) (10). Lineage 1 viruses were from the United States and Canada during 1952–2011 and lineage 2 were more recent strains from the northeastern United States. Virus isolates from Virginia were genetically similar to each other (3-bp differences) and grouped in the newly emergent lineage 2 of CVV. This monophyletic lineage shares a most recent common ancestor with a virus from Mexico isolated in 1961 (GenBank accession no. AF231118). This sister-group relationship suggests a derivation of this group from Mexico (10). However, additional historical samples from the region in Veracruz, Mexico, and elsewhere during the history of its introduction into the northeastern United States would further provide understanding of the phylogeography of the virus.
To determine vector competence of local mosquitoes for CVV, we established a laboratory strain from uninfected Ae. j. japonicus mosquitoes. Week-old female mosquitoes from the F2 generation were offered an infectious blood meal in a membrane feeder. This blood meal contained 1 mL of the CVV-4B isolate and 9 mL of sheep blood (Colorado Serum Company, Denver, CO, USA). We transferred postfeeding, engorged mosquitoes to 0.7-L cages and held them for 14 days at 25°C, a relative humidity of 75%, and a 16:8 (L:D) photoperiod and provided 10% sucrose. We measured rates of nondisseminated and disseminated infection (virus present in legs and wings) and oral transmission (virus present in saliva). We conducted this experiment 3 times.
Infectious blood meal titers ranged from 1.6 × 105 to 4.6 × 106 PFU/mL (Table 2). Ae. j. japonicus female mosquitoes were susceptible to oral infection with CVV and capable of transmitting the virus. After a 14-day incubation, CVV was present in 41% of abdomens, 38% of legs and wings, and 28% of saliva samples (Table 2). We found no significant differences among the 3 replicates for infection or transmission rates (p>0.05 by χ2 test).
Ae. j. japonicus mosquitoes are an invasive species that has spread throughout most of the eastern United States and are a competent vector of several endemic viruses (12). Although CVV was previously isolated from Ae. j. japonicus mosquitoes in the northeastern United States (4,13), we report isolation of CVV from this species in Appalachia and show that it is a competent vector of the virus. In the laboratory, vector competence of Ae. j. japonicus mosquitoes was equivalent to that for other species believed to be part of the CVV transmission cycle. For example, transmission rates for An. quadrimaculatus mosquitoes ranged from 20% to 33% after imbibing infectious blood meals with virus titers similar to those used in our study (1).
Ae. j. japonicus mosquitoes readily feed on humans and large animals, such as white-tailed deer (12). Consequently, this species probably contributes to local transmission of CVV. The study site is in close proximity to humans and pastured sheep and is frequented by deer (Figure 1). Therefore, all components for establishment of a focus of CVV are present. If Ae. j. japonicus mosquitoes are capable of transovarial transmission, as is the case with La Crosse virus, another bunyavirus (14), these mosquitoes could then contribute to concentrating the virus within this limited geographic area. Emergence of La Crosse virus in the Appalachian region of the United States has been associated with invasions by Ae. j. japonicus and Ae. albopictus mosquitoes (15). Thus, additional studies are needed to determine the role of Ae. j. japonicus mosquitoes in the transmission, maintenance, and presence of CVV.
At the time of this study, Dr. Yang was a medical entomologist and chemical ecologist in the Department of Entomology Department, Virginia Polytechnic Institute and State University, Blacksburg, VA. He is currently a microbiologist at the California Department of Public Health, Richmond, CA. His research interests are the ecology and surveillance of vectorborne diseases.
Acknowledgments
We thank Eton Bioscience, Inc. (San Diego, CA, USA) for performing sequencing.
This study was supported by the Entomology Department of Virginia Polytechnic Institute and State University. P.E.M. was supported by the Virginia Polytechnic Institute and State University, US Department of Agriculture, National Institute of Food and Agriculture Hatch Project (VA-160028).
References
- Blackmore CG, Blackmore MS, Grimstad PR. Role of Anopheles quadrimaculatus and Coquillettidia perturbans (Diptera: Culicidae) in the transmission cycle of Cache Valley virus (Bunyaviridae: Bunyavirus) in the midwest, USA. J Med Entomol. 1998;35:660–4. DOIPubMed
- Campbell GL, Mataczynski JD, Reisdorf ES, Powell JW, Martin DA, Lambert AJ, et al. Second human case of Cache Valley virus disease. Emerg Infect Dis. 2006;12:854–6. DOIPubMed
- Calisher CH, Francy DB, Smith GC, Muth DJ, Lazuick JS, Karabatsos N, et al. Distribution of Bunyamwera serogroup viruses in North America, 1956-1984. Am J Trop Med Hyg. 1986;35:429–43. DOIPubMed
- Andreadis TG, Armstrong PM, Anderson JF, Main AJ. Spatial-temporal analysis of Cache Valley virus (Bunyaviridae: Orthobunyavirus) infection in anopheline and culicine mosquitoes (Diptera: Culicidae) in the northeastern United States, 1997-2012. Vector Borne Zoonotic Dis. 2014;14:763–73. DOIPubMed
- Edwards JF. Cache Valley virus. Vet Clin North Am Food Anim Pract. 1994;10:515–24. DOIPubMed
- Nguyen NL, Zhao G, Hull R, Shelly MA, Wong SJ, Wu G, et al. Cache valley virus in a patient diagnosed with aseptic meningitis. J Clin Microbiol. 2013;51:1966–9. DOIPubMed
- Blitvich BJ, Saiyasombat R, Talavera-Aguilar LG, Garcia-Rejon JE, Farfan-Ale JA, Machain-Williams C, et al. Orthobunyavirus antibodies in humans, Yucatan Peninsula, Mexico. Emerg Infect Dis. 2012;18:1629–32. DOIPubMed
- Barker CM, Paulson SL, Cantrell S, Davis BS. Habitat preferences and phenology of Ochlerotatus triseriatus and Aedes albopictus (Diptera: Culicidae) in southwestern Virginia. J Med Entomol. 2003;40:403–10. DOIPubMed
- Kuno G, Mitchell CJ, Chang GJ, Smith GC. Detecting bunyaviruses of the Bunyamwera and California serogroups by a PCR technique. J Clin Microbiol. 1996;34:1184–8.PubMed
- Armstrong PM, Andreadis TG, Anderson JF. Emergence of a new lineage of Cache Valley virus (Bunyaviridae: Orthobunyavirus) in the Northeastern United States. Am J Trop Med Hyg. 2015;93:11–7. DOIPubMed
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42. DOIPubMed
- Kaufman MG, Fonseca DM. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae). Annu Rev Entomol. 2014;59:31–49. DOIPubMed
- Ngo KA, Maffei JG, Dupuis AP II, Kauffman EB, Backenson PB, Kramer LD. Isolation of Bunyamwera serogroup viruses (Bunyaviridae, Orthobunyavirus) in New York state. J Med Entomol. 2006;43:1004–9.PubMed
- Harris MC, Dotseth EJ, Jackson BT, Zink SD, Marek PE, Kramer LD, et al. La Crosse virus in Aedes japonicus japonicus mosquitoes in the Appalachian Region, United States. Emerg Infect Dis. 2015;21:646–9. DOIPubMed
- Leisnham PT, Juliano SA. Impacts of climate, land use, and biological invasion on the ecology of immature Aedes mosquitoes: implications for La Crosse emergence. EcoHealth. 2012;9:217–28. DOIPubMed
Figures
Tables
Cite This Article1Current affiliation: California Department of Public Health, Richmond, California, USA.






















.png)











No hay comentarios:
Publicar un comentario