
Drug Combinations to Overcome Treatment Resistance - National Cancer Institute
Identifying Novel Drug Combinations to Overcome Treatment Resistance
Many patients diagnosed with cancer have far more treatment options now than they did even a decade ago. In some cases, these treatments can produce remarkable responses, completely eradicating tumors in patients whose cancer had spread throughout their body.
But nearly all current treatments face the same problem: for many patients, they ultimately stop working. Commonly known as drug resistance, this phenomenon is one of the most challenging problems facing cancer researchers and patients today.
When cancer cells resist the effects of drugs used for treatment, they can grow and reform tumors, a process known as recurrence or relapse. Sometimes resistance develops quickly, within a matter of weeks of starting treatment. In other cases, it develops months, or even years, later.
Resistance can occur when cancer cells—even a small group of cells within a tumor—contain molecular alterations that make them insensitive to a particular drug before treatment even begins. Because cancer cells within the same tumor often have a variety of molecular alterations, this so-called intrinsic resistance is common.
In other cases of resistance, cancer cells may adapt to the drug while it is being administered, acquiring molecular changes that allow them to escape its effects.
Molecular alterations that contribute to intrinsic or acquired treatment resistance include mutation of the drug's molecular target, changes in the way the drug interacts with the tumor, broad cellular changes, and changes in the tumor microenvironment, among others. To complicate matters, many of these factors can be at play simultaneously in a single tumor.
Combining Cancer Drugs
Researchers believe one possible way to overcome or delay the development of resistance is to treat patients with combinations of different drugs.
One combination treatment approach is to "co-administer drugs that work by different molecular mechanisms," Bissan Al-Lazikani, Ph.D., of Cancer Research UK and her colleagues wrote in Nature Biotechnology, "thereby increasing tumor cell killing while reducing the likelihood of drug resistance and minimizing overlapping toxicity."
Another approach is to treat patients with drugs that block the particular mechanism of resistance their tumors have developed, and then treat them again with the drug to which they grew resistant. The idea is that this combination approach may "re-sensitize" the patients to the original treatment.
Scientists are pioneering many different methods to discover and test novel drug combinations that may be able to overcome multiple mechanisms of resistance or delay their emergence. If these efforts are successful, it could potentially transform cancer for many patients.
"If the acquisition of drug resistance that leads to treatment failure and to patient death can be substantially delayed, then cancer could become a chronic condition," Robert Brown, Ph.D., and his colleagues at the Imperial College London wrote in Nature Reviews Cancer.
Keeping Cancer Drugs inside Cells
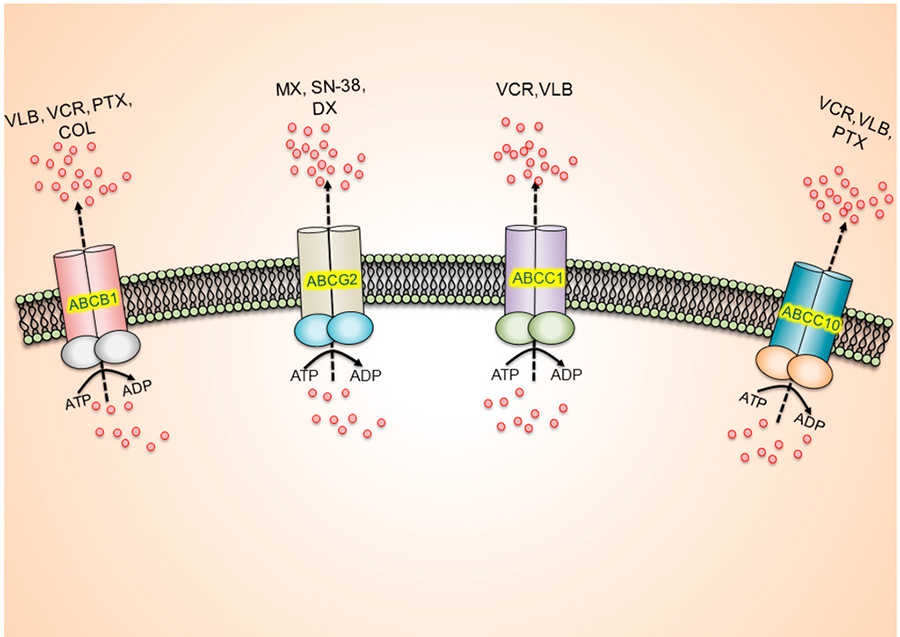
One mechanism by which cancer cells resist treatment is by expelling cancer drugs. For example, healthy cells have proteins known as transporters that pump out toxic agents. One such group of proteins, called the ATP-binding cassette (ABC) transporters, is able to expel some chemotherapy drugs, including doxorubicin, and some targeted therapies, like imatinib (Gleevec®).
Michael Gottesman, M.D., head of the multidrug resistance section of NCI's Center for Cancer Research, and his colleagues study how ABC transporters contribute to cancer drug resistance. More than 30 years ago, they discovered that "in some cases, when patients go from being sensitive to resistant to treatment, their cancer cells start to overexpress ABC transporters," he said.
In more recent studies, they analyzed DNA and RNA from tumors that are sensitive to or resistant to chemotherapy and found "increases in expression of one or more of these transporters in many different kinds of tumors," said Dr. Gottesman.
When given in combination with other cancer therapies, drugs that block the activity of ABC transporters might allow greater amounts of anticancer drugs to accumulate in cancer cells, thereby boosting their effect, he explained.
Earlier versions of ABC transporter inhibitors did not hit their intended molecular targets specifically and caused serious side effects. A later generation of inhibitors were better at blocking the activity of these transporters but were also very toxic in clinical trials. Toxicity likely stems from the fact that ABC transporters also serve important roles in healthy cells, Dr. Gottesman explained.
Now scientists are working to develop the next generation of ABC transporter inhibitors that, they hope, strike a balance between increased efficacy and decreased toxicity. For example, several independent groups have found that the drug osimertinib (Tagrisso®), originally developed as a tyrosine kinase inhibitor, can block ABC transporters and enhance the effect of chemotherapy drugs in mice. Importantly, one study showed that mice treated with osimertinib did not demonstrate weight loss, a sign of toxicity.
Researchers also plan to take a precision medicine approach when designing clinical trials for new inhibitors by using genetic profiling to help determine which patients' tumors overexpress ABC transporters and are thus most likely to benefit from treatment that includes an ABC transporter inhibitor.
Erasing Reversible Modifications
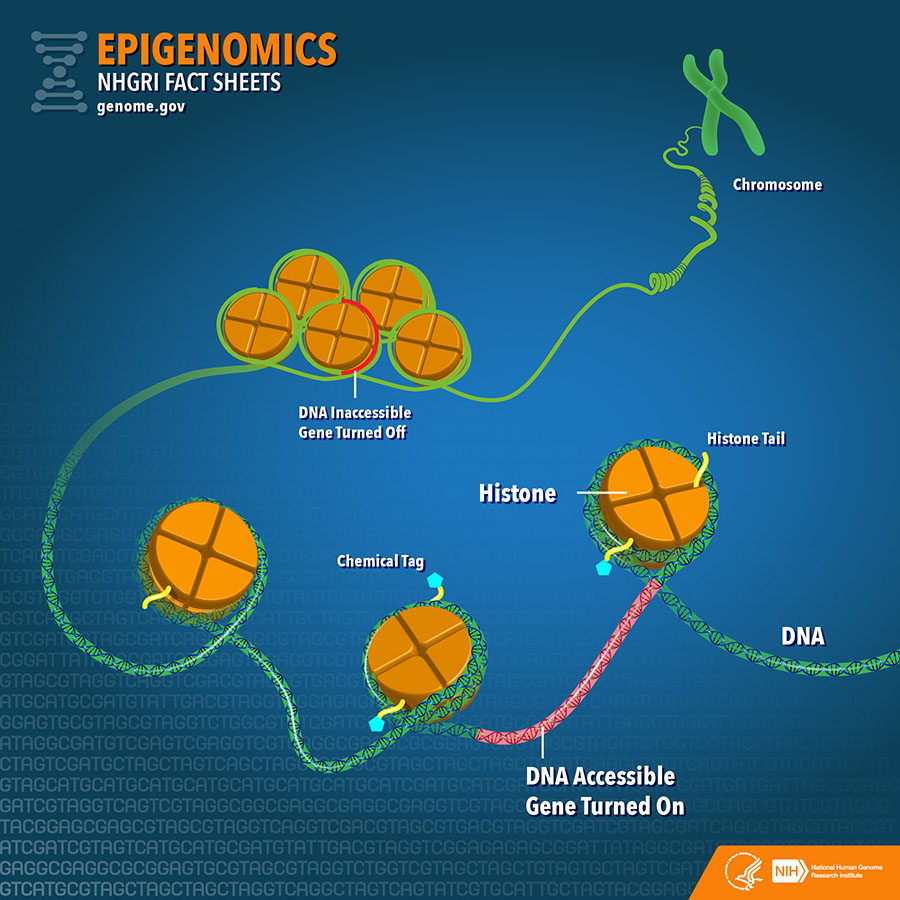
In addition to arising through genetic alterations, drug resistance can also emerge as a result of alterations in cancer cells' epigenetic codes—molecular modifications that, without altering the DNA code, turn genes on or off. A major difference between epigenetics and genetics is that the epigenetic code is reversible and can fluctuate over the course of a cell’s lifetime.
One type of epigenetic alteration, called DNA methylation, occurs when enzymes attach chemical tags named methyl groups to DNA. Another type, termed histone modification, occurs when enzymes attach chemical tags to histones, proteins that are involved in "packaging" DNA into compact structures. Both DNA methylation and histone modifications can turn nearby genes on or off.
Studies have shown that cancer cells can acquire epigenetic alterations that allow them to resist treatment. For example, more than 15 years ago, Dr. Brown and his colleagues found that, after chemotherapy treatment, 25%-35% of patients with ovarian cancer acquire high levels of methylated DNA near the hMLH1 gene, and that this epigenetic alteration predicts poor survival for patients with ovarian cancer.
In subsequent studies, the researchers showed that treating mice bearing cisplatin (Platinol®)-resistant ovarian tumors with decitabine (Dacogen®)—a drug that inhibits DNA methylation—reversed hMLH1 methylation and re-sensitized the tumors to cisplatin. Furthermore, a recent study comparing DNA methylation across the genome of several cisplatin-sensitive and -resistant ovarian cancer cell lines confirmed that methylation of hMLH1, among other genes, may cause cisplatin resistance.
And there’s some evidence that using drugs that erase the epigenetic alterations that drive resistance might restore sensitivity to chemotherapy in patients. In one phase II clinical trial involving patients whose ovarian cancer was resistant to chemotherapy, delivering decitabine prior to treatment with carboplatin (Paraplatin®) led to tumor shrinkage in 35% of patients. Historically, only about 10% of such patients given carboplatin alone respond to treatment, suggesting that prior treatment with decitabine may have increased the patients’ sensitivity to chemotherapy.
In several other preliminary trials, prior treatment with epigenetic inhibitors temporarily reversed resistance to chemotherapy.
"Given all of these emerging data, it appears that epigenetic agents may have the potential to help overcome chemotherapy resistance and improve sensitivity to chemotherapy," Julius Strauss, M.D., and William Figg, Sr., Pharm.D., of NCI's Center for Cancer Research, wrote in Anticancer Research.
Altering the Tumor Microenvironment
Immunotherapies—therapies that help the immune system to fight cancer—have generated robust and durable responses in patients with various types of cancer. But, like other therapies, some patients are inherently resistant to immunotherapies and some patients who initially respond to immunotherapy eventually acquire resistance.
Researchers hope that combining immunotherapies with other immunotherapies or with different types of cancer drugs may counteract the development of resistance. For example, a team led by Taha Merghoub, Ph.D., and Jedd Wolchok, M.D., Ph.D., of Memorial Sloan Kettering Cancer Center, recently discovered a drug combination that can overcome a form of resistance to immune checkpoint inhibitors in mice that results from the presence of myeloid cells in the tumor microenvironment. While immune checkpoint inhibitors enable T cells to recognize and kill cancer cells, myeloid cells counteract their effect by inactivating T cells.
When the researchers treated mice bearing checkpoint inhibitor-resistant melanoma or breast cancer tumors with the combination of two checkpoint inhibitors and a drug that prevents myeloid cells from inactivating T cells, tumor growth was substantially delayed and greater numbers of T cells were activated in the tumor microenvironment than in mice treated with the two checkpoint inhibitors alone.
The researchers are now testing this combination in an early-stage trial of patients with solid tumors.
Simultaneously Testing Combinations
Although different mechanisms of resistance are usually studied separately, there is a consensus among researchers that multiple mechanisms of resistance are likely at play in a single tumor. So it's likely that each patient will require a unique approach to overcome drug resistance, said Dr. Figg.
"We talk about personalizing cancer treatment, but I think personalizing treatment to overcome drug resistance is also an important concept," echoed Dr. Gottesman.
But the enormous number of drug combinations that would need to be tested to find the best match for each patient is a daunting impediment to this precision approach. To overcome this hurdle, researchers are developing methods to systematically evaluate large numbers of potential drug combinations.
One approach is to simultaneously test the effects of multiple combinations of FDA-approved drugs on cancer cell lines—a method called high-throughput screening. This is the technique that a team led by Cyril Benes, Ph.D., of Massachusetts General Hospital Cancer Center, and Jeffrey Engelman, M.D., Ph.D., vice president and global head of oncology at Novartis, used to assess the ability of drug pairs to overcome drug resistance in lung cancer.
They performed the high-throughput screen on 60 lung cancer cell lines they developed from patients whose lung cancer had become resistant to targeted therapy. They tested the killing effect of a panel of 76 different targeted therapies alone or in combination with the original drug to which the patients had become resistant.
For each cell line, the researchers identified an average of 3.4 drug combinations that effectively killed the cancer cells. For many of the effective drug pairs, they realized that the second drug inhibited a signaling pathway that was able to bypass the effect of the original drug. They also demonstrated that treatment with only one drug of the pair was ineffective because, the study authors believe, the alternate signaling pathway was able to compensate.
"When cancer cells are molecularly rewired" and acquire resistance after treatment," said Dr. Benes, "we can try to cut off these other routes that reactivate key pro-survival signals" by treating with drug combinations.
Based on these results, the researchers speculated that a similar test could be developed and used on patient tumor samples in the clinic to help identify drug combinations that are likely to overcome resistance among individual patients.
I think the next frontier in precision genomic medicine is figuring out how to circumvent resistance.
"I think the next frontier in precision genomic medicine is figuring out how to circumvent resistance," said Laurie Glimcher, M.D., president and CEO of Dana-Farber Cancer Institute, at a lecture at the National Institutes of Health on November 7, 2016.
"To me the biggest gap we have is figuring out how to do that kind of combination therapy ex vivo in human cells, so we don't have to do thousands of clinical trials," she continued. "We have to figure out, using organoids or other systems yet to be discovered, how we can most quickly evaluate each patient's response" to combinations of therapies.
NCI Drug Formulary
One of the challenges of drug combination research is acquiring permission to test therapeutic agents developed by different companies. The NCI Drug Formulary, an initiative designed in partnership with pharmaceutical companies, biotechnology companies, and academic investigators, aims to make it easier for investigators to access and test combinations of early stage therapeutics developed by different companies. Through the formulary, researchers will be able to request drugs from a centralized formulary list with an abbreviated approval process. NCI will act as an intermediary and will leverage existing physical and support infrastructure to reduce costs, allowing for a single agreement and approval process that will provide researchers with access to multiple investigational agents.
























.png)











No hay comentarios:
Publicar un comentario