
11/0/2016
The Food and Drug Administration (FDA) has granted accelerated approval to olaratumab (Lartruvo®) for the treatment of some patients with soft tissue sarcoma.The approval is for the use of olaratumab in combination with doxorubicin in patients with sarcoma that cannot be cured by radiation therapy or surgery and for whom anthracycline-based chemotherapy would be an appropriate treatment.
Olaratumab Approved to Treat Advanced Soft Tissue Sarcoma
November 2, 2016 by NCI Staff
The Food and Drug Administration (FDA) has granted accelerated approval to olaratumab (Lartruvo®) for the treatment of some patients with soft tissue sarcoma.
The approval is for the use of olaratumab in combination with doxorubicin in patients with sarcoma that cannot be cured by radiation therapy or surgery and for whom anthracycline-based chemotherapy would be an appropriate treatment.
The standard treatment for soft tissue sarcoma for decades has been doxorubicin alone or in combination with other treatments.
“This is the first new approval for the initial treatment of soft tissue sarcoma in 40 years, and this is significant,” said Alice Chen, M.D., of NCI’s Developmental Therapeutics Clinic in the Division of Cancer Treatments and Diagnosis. “Sarcoma is a group of diseases that has not benefited from research advances as much as some other types of cancer.”
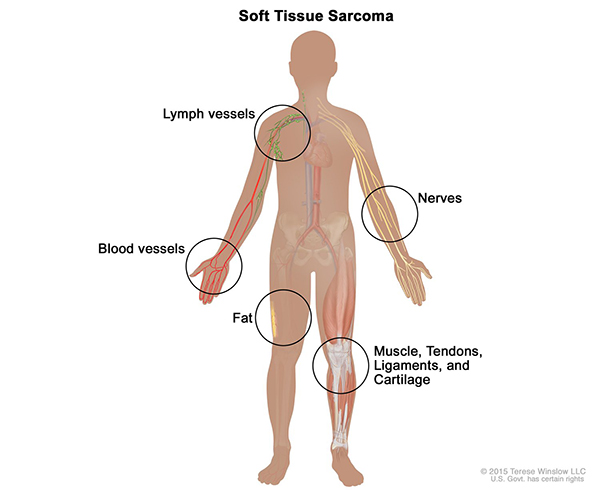
Soft tissue sarcoma forms in the soft tissues of the body, including muscle, tendons, fat, lymph vessels, blood vessels, nerves, and tissue around joints. The tumors can be found anywhere in the body, but they often form in the arms, legs, chest, and abdomen.
Clinical Trial Results
Olaratumab is a monoclonal antibody that prevents a protein called platelet-derived growth factor (PDGF) receptor-alpha from binding with several ligands on cancer cells. By disrupting this binding process, the drug may help block growth-promoting signals in cancer cells.
The FDA approval was based on a randomized phase II clinical trial that included 133 patients aged 18 years or older with more than 25 different subtypes of metastatic soft tissue sarcoma. Patients in the trial received either olaratumab plus doxorubicin or doxorubicin alone, and the primary endpoint was progression-free survival.
Median progression-free survival was 6.6 months for patients who received olaratumab plus doxorubicin and 4.1 months for those who received doxorubicin alone. The median overall survival was 26.5 months in the olaratumab group versus 14.7 months in the doxorubicin-alone group.
Gary K. Schwartz, M.D., of Columbia University Medical Center and his colleagues reported the trial results earlier this year in The Lancet.
Given the “desperate need of patients with soft-tissue sarcomas for new active drugs,” the trial results are “promising but need confirmation in a larger study,” wrote Winette van der Graaf, M.D., Ph.D., of the Institute of Cancer Research, London, in an accompanying editorial.
A study known as ANNOUNCE is currently recruiting patients with advanced soft tissue sarcoma. The trial, which will compare gemcitabine (Gemzar®) and docetaxel (Taxotere®)alone or in combination with olaratumab, will measure overall survival as a primary endpoint.
The FDA’s accelerated approval requires olaratumab’s maker, Eli Lilly, to complete a randomized phase III trial that confirms the safety and efficacy of the combination therapy for patients with soft tissue sarcoma.
Side Effects
Olaratumab has “serious risks,” according to the FDA, including infusion-related reactions, such as low blood pressure, fever, chills, and rash. Among the most common side effects of olaratumab are nausea, fatigue, low levels of white blood cells (neutropenia), musculoskeletal pain, and inflammation of the mucous membranes (mucositis).
In the phase II trial, however, the side effects associated with the combination therapy were not significantly worse than those associated with doxorubicin alone, noted Dr. Chen. Doxorubicin has been used for decades, and doctors are familiar with managing its side effects, she added.
Accelerating Approval
NCI’s Translational Research Program—through its Specialized Program of Research Excellence (SPORE)—supported some of the early studies that explored the vulnerabilities of soft tissue sarcoma cells that could potentially be targeted by a new therapy.
“At the Translational Research Program, we are always excited when a drug makes it from laboratory studies into clinical trials and is approved for patients,” said Peter Ujhazy, M.D., Ph.D., of the Translational Research Program, which is part of NCI’s Division of Cancer Treatment and Diagnosis.
“But this will not be the end of the story,” Dr. Ujhazy continued. “Researchers can now look for new therapeutic targets in these cancers, new treatment combinations, and mechanisms of resistance.”
To assess the combination therapy in patients, Dr. Schwartz and his colleagues used a combination phase I/II clinical trial: Once a safe dose had been determined in the phase I study, the phase II trial to assess the drug’s efficacy was opened.
NCI is increasingly supportive of combining phase I/II trials because of the potential to shorten the time an active drug is in development before FDA approval, noted Dr. Chen.
“In this case, based on the early hint of a benefit in the phase II trial, we now have a new treatment option for patients with soft tissue sarcoma,” she added.






















.png)












No hay comentarios:
Publicar un comentario