Precision Oncology: Nanoparticles Target Bone Cancers in Dogs

Caption: Veterinary researcher Timothy Fan with his healthy family pet Ember.
Credit: L. Brian Stauffer
Credit: L. Brian Stauffer
Many people share their homes with their pet dogs. Spending years under the same roof with the same environmental exposures, people and dogs have something else in common that sometimes gets overlooked. They can share some of the same diseases, such as diabetes and cancer. By studying these diseases in dogs, researchers can learn not only to improve care for people but for their canine friends as well.
As a case in point, an NIH-funded team of researchers recently tested a new method of delivering chemotherapy drugs for osteosarcoma, a bone cancer that affects dogs and people, typically teenagers and older adults. Their studies in dogs undergoing treatment for osteosarcoma suggest that specially engineered, bone-seeking nanoparticles might safely deliver anti-cancer drugs precisely to the places where they are most needed. These early findings come as encouraging news for the targeted treatment of inoperable bone cancers and other malignancies that spread to bone.
Nanoparticles are engineered in the lab by manipulating matter on an atomic and molecular scale into custom-made, three-dimensional materials that measure under 100 nanometers (a nanometer is 1 billionth of a meter). These materials can be programmed to seek out something unique about a tissue and bypass other parts of the body. Cancer researchers seek to utilize this homing ability to deliver a chemotherapy drug directly to a patient’s tumor, boosting its effectiveness and limiting its side effects.
But the development of nanoparticles to fight cancer has been slowed by some natural limitations of testing them in mice, the preferred mammalian research model. One is scale. For a nano-sized drug delivery system to work in people with advanced cancer, it must penetrate tumors much larger than those in any mouse. Another issue is the tumors in mice don’t often occur spontaneously as they do in people. Researchers typically induce them by injecting human cells into mice with compromised immune systems to avoid rejection.
As published recently in Proceedings of the National Academy of Sciences, veterinary researcher Timothy Fan and materials scientist Jianjun Cheng, both at the University of Illinois at Urbana-Champaign, teamed up to overcome these obstacles. They did it by taking a nanoparticle developed in Cheng’s lab and testing it in pet dogs being treated for this cancer at Fan’s veterinary research clinic.
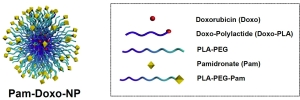
Caption: Pam-Doxo nanoparticles (left). Color-coded parts of the nanoparticle (right)
Credit: Yin Q and Tang L et al., 2016
Credit: Yin Q and Tang L et al., 2016
Here’s how the process worked. Cheng and his colleagues began in the lab constructing the virus-sized nanoparticles by fabricating polymers and attaching to them trace amounts of the chemotherapy drug doxorubicin (Doxo), which is used to treat many cancers including osteosarcoma. They then coated the polymers with a drug known as pamidronate (Pam). Pam is attracted to areas of active bone repair, which is the response in dogs and people when osteosarcoma invades and destroys bone tissue.
The researchers first confirmed that their Pam-coated nanoparticles bind to hydroxyapatite, a major inorganic component of bone. They then showed that the addition of Pam to their drug-laden nanoparticles also improved their ability to stick to lab dishes that mimic the surface of bone, resulting in improved killing of osteosarcoma cells. Their Pam-Doxo nanoparticles also concentrated in the cancer-ridden bones of mice with osteosarcoma, slowing tumor growth and minimizing bone loss.
To help Cheng bring his Pam-Doxo nanoparticle from lab to clinic, Fan and his colleagues enrolled—with the consent of their owners—nine dogs undergoing treatment for osteosarcoma at the University of Illinois Veterinary Teaching Hospital. The dogs in this Phase I safety trial weighed from 88 to 132 pounds, putting them within a comparable skeletal size range of a child or young adult with osteosarcoma.
The researchers showed in the dogs that Cheng’s nanoparticles accumulated in each bone tumor within two hours of intravenous infusion. Importantly, the dogs tolerated doses of the nanoparticle treatment equivalent to what might be given to a person with no signs of toxicity, as would be hoped with a targeted therapy.
Three weeks after the nanoparticle infusion, the dogs had their diseased limbs amputated, the standard of care for treating canine osteosarcoma. While this early clinical trial was designed primarily to test safety, studies of each dog’s amputated limb revealed dead or dying tumor tissue in the bone.
The next challenge will be to ramp up production of the Pam-Doxo nanoparticle and enable its continued clinical study. The nanoparticles used in this study were painstakingly produced in relatively small quantities by a couple of graduate students in the lab.
That aside, the findings offer proof-of-concept that nanoparticles could be used to target bone cancers in large mammals, including humans. The approach may one day be applied to treat metastatic bone cancers, which can be widely distributed throughout the body and impossible to remove with surgery. If so, this research would be good news for humans and, importantly, bring improved care to their four-legged “best friends” with cancer.
Reference:
[1] Pamidronate functionalized nanoconjugates for targeted therapy of focal skeletal malignant osteolysis. Yin Q, Tang L, Cai K, Tong R, Sternberg R, Yang X, Dobrucki LW, Borst LB, Kamstock D, Song Z, Helferich WG, Cheng J, Fan TM. Proceedings of the National Academy of Sciences. July 25, 2016. [Epub ahead of print]
Links:
Bone Cancer (National Cancer Institute/NIH)
Cheng Research Group (University of Illinois, Urbana-Champaign)
Timothy Fan (University of Illinois, Urbana-Champaign)
NIH Support: National Cancer Institute; Common Fund






















.png)











No hay comentarios:
Publicar un comentario