07/28/2016 09:00 AM EDT

For Gregory Schwartz, working in total darkness has its benefits. Only in the pitch black can Schwartz isolate resting neurons from the eye’s retina and stimulate them with their natural input—light—to get them to fire electrical signals. Such signals not only provide a readout of the intrinsic properties of each neuron, but information that enables […]
Creative Minds: Reverse Engineering Vision
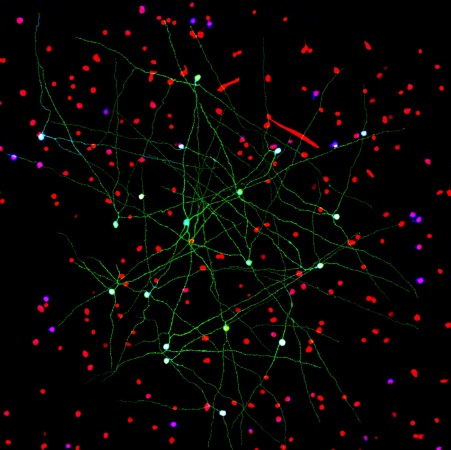
Caption: Networks of neurons in the mouse retina. Green cells form a special electrically coupled network; red cells express a distinctive fluorescent marker to distinguish them from other cells; blue cells are tagged with an antibody against an enzyme that makes nitric oxide, important in retinal signaling. Such images help to identify retinal cell types, their signaling molecules, and their patterns of connectivity.
Credit: Gregory Schwartz, Northwestern University
Credit: Gregory Schwartz, Northwestern University
For Gregory Schwartz, working in total darkness has its benefits. Only in the pitch black can Schwartz isolate resting neurons from the eye’s retina and stimulate them with their natural input—light—to get them to fire electrical signals. Such signals not only provide a readout of the intrinsic properties of each neuron, but information that enables the vision researcher to deduce how it functions and forges connections with other neurons.
The retina is the light-sensitive neural tissue that lines the back of the eye. Although only about the size of a postage stamp, each of our retinas contains an estimated 130 million cells and more than 100 distinct cell types. These cells are organized into multiple information-processing layers that work together to absorb light and translate it into electrical signals that stream via the optic nerve to the appropriate visual center in the brain. Like other parts of the eye, the retina can break down, and retinal diseases, including age-related macular degeneration, retinitis pigmentosa, and diabetic retinopathy, continue to be leading causes of vision loss and blindness worldwide.
In his lab at Northwestern University’s Feinberg School of Medicine, Chicago, Schwartz performs basic research that is part of a much larger effort among vision researchers to assemble a parts list that accounts for all of the cell types needed to make a retina. Once Schwartz and others get closer to wrapping up this list, the next step will be to work out the details of the internal wiring of the retina to understand better how it generates visual signals. It’s the kind of information that holds the key for detecting retinal diseases earlier and more precisely, fixing miswired circuits that affect vision, and perhaps even one day creating an improved prosthetic retina.

Gregory Schwartz
In anticipation of the extremely challenging job ahead, Schwartz received a 2015 NIH Director’s New Innovator Award to develop improved strategies to map retinal circuits and determine their functions. Schwartz focuses most of his attention on the retina’s fifth and innermost layer, which is composed primarily of neurons called ganglion cells. Although the count continues, it is estimated that there are more than 40 ganglion cell types. These cells self-organize into circuits that specialize in extracting a specific feature of a visual signal and processing it further. This process is complex–and amazing. In fact, Schwartz calculated these cells continuously stream every point in visual space to the brain from 150 different visual pathways. Each pathway projects specific information, such as shape, color, contrast, motion, direction, and location.
This year, Schwartz and Amurta Nath, a Ph.D. student at Northwestern, reported discovering two novel types of retinal ganglion cells that select orientation—that is, they inform the brain whether an object is positioned vertically or horizontally [1]. Interestingly, neuroscientists David Hubel and Torsten Wiesel won the 1981 Nobel Prize in Physiology or Medicine for discovering selective orientation in the brain’s cerebral cortex. The latest discovery shows that the retina streams a parallel orientation signal to the brain.
With his New Innovator Award, Schwartz and his colleagues will map out strategies to take apart and put back together, or “reverse engineer,” various parts of the mouse retina. The first step in the process is to devise methods for determining how ganglion cells interact, as well as how various retinal cell types connect to form circuits. Creating this “connectivity matrix” will involve precise labeling of the various cells and synapses, and Schwartz is already hammering away on the methodology.
For Schwartz, step two will be to move beyond a purely anatomical description of the cells and begin defining how these multi-component circuits actually function. The goal is to be in a position one day to shine a defined pattern of light on individual cells within a circuit, and predict accurately the strength and duration of the electrical signal that each cell will generate.
Some very sophisticated computational modeling will be required to achieve that feat. Schwartz, a computational biologist by training, thinks that such work must be grounded in a detailed understanding of the different cell types, their physiology, and their often complex, non-linear signaling behavior. While this bottom-up approach to computation is challenging, Schwartz thinks that it is the only way to get the math right.
During his postdoctoral training, Schwartz at first wanted to apply his computational skills to map the brain with its tens of billions of cells and connections. Schwartz quickly realized what a tall task that would be. Then, during a required rotation in a vision research lab, he became fascinated by the retina and the knowledge that can be obtained by studying a much smaller, simpler neural system—one whose only input is light and only output funnels predictably to the optic nerve. Unlike mapping the entire brain, Schwartz decided that mapping the retina was something that he couldrealistically hope to be part of accomplishing in the coming years.
I certainly hope that his hunch is correct and that his retina mapping work, along with that of many other NIH-supported researchers, charts the way for new strategies for helping the millions of people whose vision is threatened or impaired by retinal problems. And if this kind of detailed neuronal mapping can be achieved for the retina, it will provide further momentum for the effort to identify and map all of the circuits in the human brain—a specific goal of the U.S. Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative.
Reference:
[1] Cardinal orientation selectivity Is represented by two distinct ganglion cell types in mouse retina. Nath A, Schwartz GW. J Neurosci. 2016 Mar 16;36(11):3208-3221.
Links:
Retinal Disease (National Eye Institute/NIH)
Schwartz Lab (Feinberg School of Medicine, Northwestern University, Chicago)
Schwartz NIH Project Information (NIH RePORTER)
NIH Director’s New Innovator Award (Common Fund)
NIH Support: Common Fund; National Eye Institute





















.png)
.png)











No hay comentarios:
Publicar un comentario