
Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19
Decision Memo
Updated May 3, 2020
In the context of community transmission where continued testing is impractical, available evidence at this time indicates that an interim strategy based on time-since-illness-onset and time-since-recovery can be implemented to establish the end of isolation. Practical application of a symptom-based strategy cannot prevent all infections.
At this time, data are limited regarding how long persons shed infectious SARV-CoV-2 RNA after infection. Key findings are summarized here.
- Viral burden measured in upper respiratory specimens declines after onset of illness (CDC unpublished data, Midgely 2020, Young 2020, Zou 2020, Wölfel 2020).
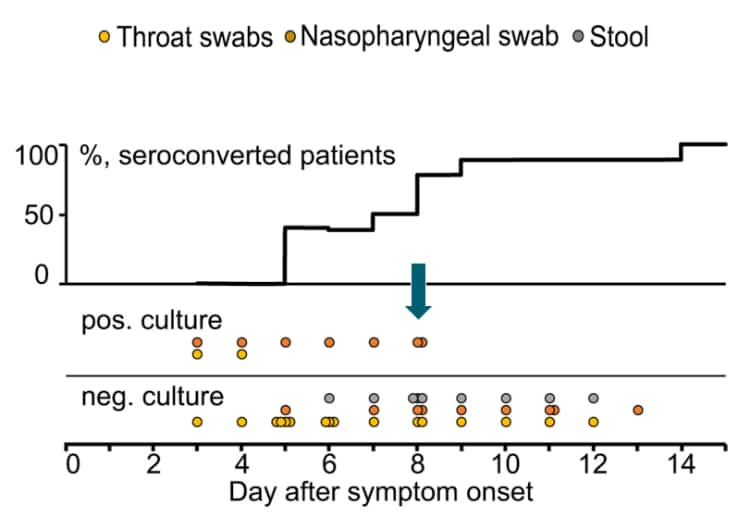
- At this time, replication-competent virus has not been successfully cultured more than 9 days after onset of illness. The statistically estimated likelihood of recovering replication-competent virus approaches zero by 10 days (CDC unpublished data, Wölfel 2020, Arons 2020).
- As the likelihood of isolating replication-competent virus decreases, anti-SARS-CoV-2 IgM and IgG can be detected in an increasing number of persons recovering from infection (Wölfel 2020).
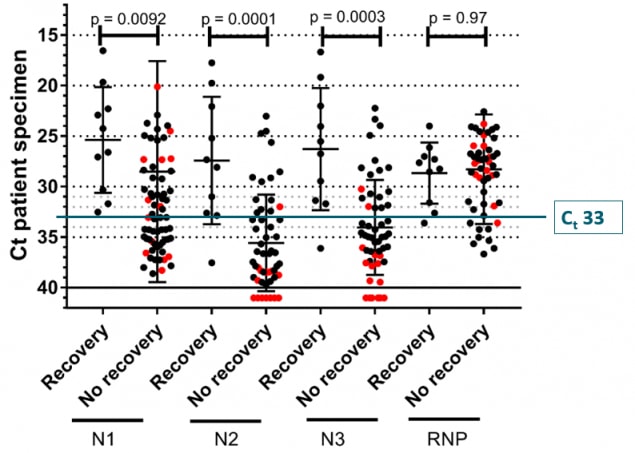
- Attempts to culture virus from upper respiratory specimens have been largely unsuccessful when viral burden is in low but detectable ranges (i.e., Ct values higher than 33-35[1])(CDC unpublished data).
- Following recovery from clinical illness, many patients no longer have detectable viral RNA in upper respiratory specimens. Among those who continue to have detectable RNA, concentrations of detectable RNA 3 days following recovery are generally in the range at which replication-competent virus has not been reliably isolated by CDC (CDC unpublished data, Young 2020).
- No clear correlation has been described between length of illness and duration of post-recovery shedding of detectable viral RNA in upper respiratory specimens (CDC unpublished data, Midgely 2020, Wölfel 2020).
- Infectious virus has not been cultured from urine or reliably cultured from feces (CDC unpublished data, Midgely 2020, Wölfel 2020); these potential sources pose minimal if any risk of transmitting infection and any risk can be sufficiently mitigated by good hand hygiene.
For an emerging pathogen like SARS-CoV-2, the patterns and duration of illness and infectivity have not been fully described. However, available data indicate that shedding of SARS-CoV-2 RNA in upper respiratory specimens declines after onset of symptoms. At 10 days after illness onset, recovery of replication-competent virus in viral culture (as a proxy of the presence of infectious virus) is decreased and approaches zero. Although persons may produce PCR-positive specimens for up to 6 weeks (Xiao, 2020), it remains unknown whether these PCR-positive samples represent the presence of infectious virus. After clinical recovery, many patients do not continue to shed SARS-CoV-2 viral RNA. Among recovered patients with detectable RNA in upper respiratory specimens, concentrations of RNA after 3 days are generally in ranges where virus has not been reliably cultured by CDC. These data have been generated from adults across a variety of age groups and with varying severity of illness. Data from children and infants are not presently available.
Epidemiologists rely on surrogate evidence, such as that presented here, to approximate when and for how long someone may be infectious to others. Although a precise estimate of this residual risk of transmission after recovery from COVID-19 illness cannot be generated at this time, it is likely substantially less than the risk during illness when available data suggest most person-to-person transmissions occurs.
This change increases the period of recommended isolation by 3 days, from 7 to 10 days. This more cautious approach is intended to more stringently limit transmissions that may occur from persons following recovery from illness and thereby enhance ongoing efforts to control COVID-19 illness. Although this change increases an individuals’ isolation period, as illness incidence decreases it will affect fewer persons and thereby limit overall societal burden of time spent in isolation.
While this strategy can apply to most recovered persons, CDC recognizes there are circumstances under which there is an especially low tolerance for post-recovery SARS-CoV-2 shedding and risk of transmitting infection.[2] In such circumstances, employers and local public health authorities may choose to apply more stringent recommendations, such as a test-based strategy, if feasible, or a requirement for a longer period of isolation after illness resolution. Entities enacting such policies should do so explicitly, with clear justification, and in coordination with local public health authorities.
There are risks and benefits to any policy decision. These recommendations are based on the best information available at this time and the practical realities of an evolving pandemic. No policy decision will result in 100% certainty that all recovered individuals are no longer infectious.
CDC will continue to closely monitor the evolving science for any signals that would warrant reconsideration of this policy.
Recommendation: For persons recovered from COVID-19 illness, CDC recommends that isolation be maintained for at least 10 days after illness onset and at least 3 days (72 hours) after recovery. Illness onset is defined as the date symptoms begin. Recovery is defined as resolution of fever without the use of fever-reducing medications with progressive improvement or resolution of other symptoms. Ideally, isolation should be maintained for this full period to the extent that it is practicable under rapidly changing circumstances.
While this strategy can apply to most recovered persons, either a test-based strategy (if feasible) or a symptom-based strategy with more stringent requirements may be used for recovered persons for whom there is low tolerance for post-recovery SARS-CoV-2 shedding and infectious risk because they are:
- Persons who could pose a risk of transmitting infection to
- Vulnerable individuals at high risk for morbidity or mortality from SARS-CoV-2 infection, or
- Persons who support critical infrastructure
- Persons normally residing in congregate living facilities (e.g., correctional/detention facilities, retirement communities, ships) where there might be increased risk of rapid spread and morbidity or mortality if spread were to occur.
- Persons who because they are immunocompromised[1] may have prolonged viral shedding.
The correlation presented here between Ct values and the ability to recover replication-competent virus is presently only applicable to upper respiratory specimens (mostly nasopharyngeal swabs) that have been assayed at CDC. These data are presented as one of a variety of pieces of evidence to inform this interim guidance. This relationship should not be inferred to apply to Ct values obtained by other laboratories or used to define a strict Ct cutoff. At this time, Ct values should not be used to define infectiousness or demonstrate the absence of risk for transmission.
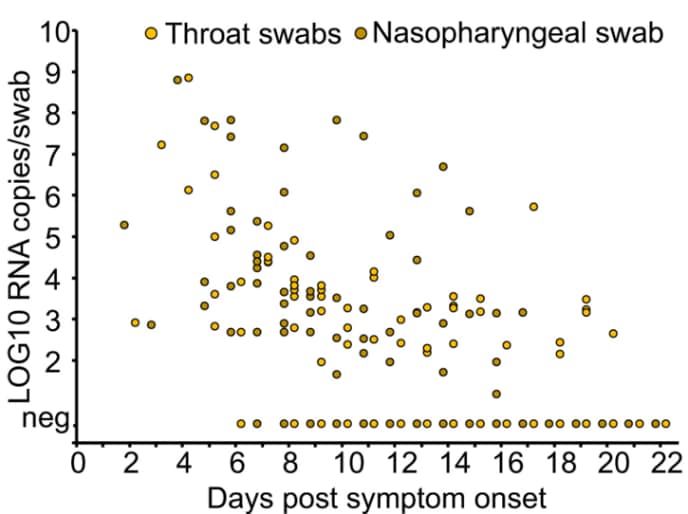
Figure 1: From Wölfel et al. demonstrating declining viral burden in upper respiratory specimens as illness progresses and decreasing capacity to isolate replication-competent virus from these same specimens as the number of patients with detectable IgM and IgG increases.
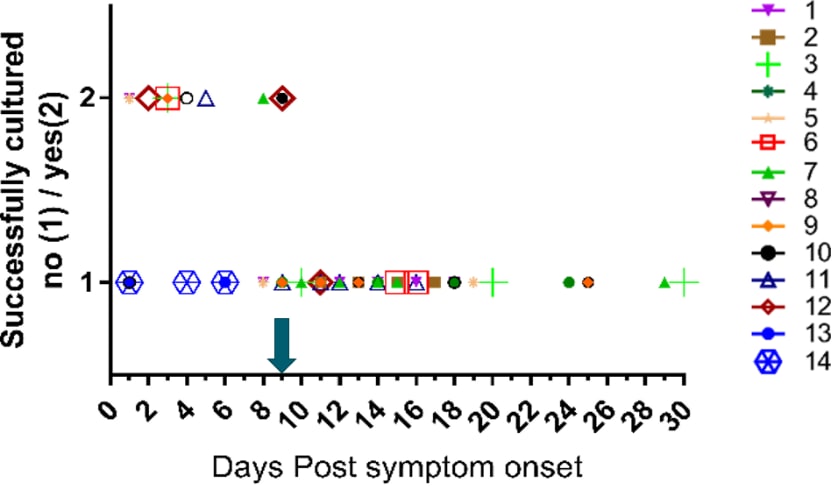
Figure 2: From Midgely et al. demonstrating inability to recover replication-competent virus from specimens collected more than 9 days after illness onset. Kaplan-Meier analysis shows time to inability to recover replication-competent SARS-CoV-2 from 14 U.S. patients. Last probability of successful isolation falls to 50% at day 4 after illness onset and to 20% at day 8. After day 9, probability approaches zero. Unpublished CDC data.
Figure 3: CDC unpublished data showing median Ct values and their 95% confidence intervals among specimens from which replication-competent virus was recovered and not recovered according to the Ct value for the amplification target (N1, N2, or N3) in the CDC RT-PCR assay. RNP = human RNase P, a positive control for the presence of adequate human sample. Red dots indicate specimens with inconclusive RT-PCR amplification according to their corresponding Ct values and culture results.
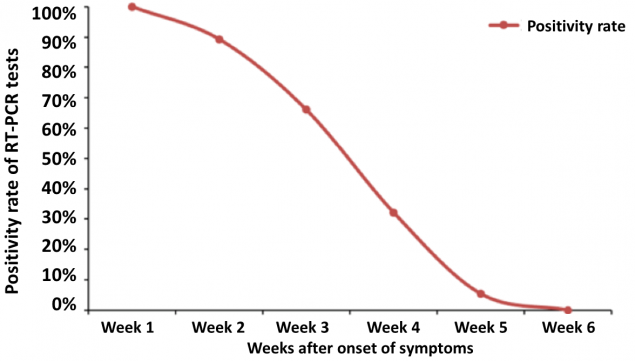
Figure 4: From Xiao et al. demonstrating persistence of RT-PCR-positive nasal swabs in 56 patients who experienced mild to moderate illness (as defined by Chinese guidelines). The positive rate was highest at week 1 (100%), followed by 89.3%, 66.1%, 32.1%, 5.4% and 0% at week 2, week 3, week 4, week 5 and week 6 respectively.
Footnotes:
1 Ct = cycle threshold, a value that is inversely related to viral burden – higher Ct values represent lower viral burden.
2 Persons who could pose a risk of transmitting infection to: 1) Vulnerable individuals at high-risk for morbidity or mortality from SARS-CoV-2 infection; 2) Persons who are critical to the maintenance of social infrastructure; or, 3) Persons who work with high-value human assets. Residents of congregate living facilities (e.g., correctional/detention facilities, retirement communities, ships) where there might be increased risk of transmission and/or morbidity and mortality from infection. Persons who are immunocompromised for whom there is ample experience that immunocompromising conditions can prolong the time that affected persons shed a variety of viral and bacterial pathogens longer than other persons with fully functional immunity, and that until the course of COVID-19 illness and shedding of SARS-CoV-2 is better understood in this population, caution is warranted.
References:
- Arons MM, Hatfield KM, Redd SC, Kimball A, James A, Jacobs JR, et al. (2020). Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. doi:10.1056/NEJMoa2008457.
- Midgley CM, Kujawski SA, Wong KK, Collins, JP, Epstein ., Killerby ME et al. (2020). Clinical and Virologic Characteristics of the First 12 Patients with Coronavirus Disease 2019 (COVID-19) in the United States. Nature Medicine, in print.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. (2020). Virological assessment of hospitalized patients with COVID-2019. Nature. doi:10.1038/s41586-020-2196-x
- Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients [published online ahead of print, 2020 Apr 19]. Clin Infect Dis. 2020; ciaa460. doi:10.1093/cid/ciaa460
- Young BE, Ong SWX, Kalimuddin S, Low JG, Ta, SY, Loh J, et al. (2020). Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. doi:10.1001/jama.2020.3204
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. (2020). SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med, 382(12), 1177-1179. doi:10.1056/NEJMc200173






















.png)












No hay comentarios:
Publicar un comentario