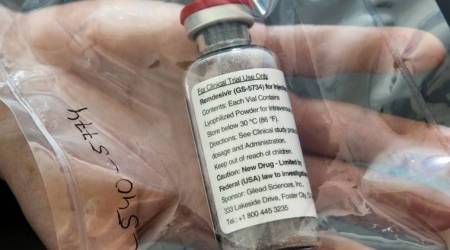
US pharma giant Gilead Sciences seeks marketing authorisation from India for remdesivir
Gilead Sciences, who is the patent holder of the drug, has the complete data about the pre-clinical and clinical studies for remdesivir, sources said. The medicine has been issued an Emergency Use Authorization (EUA) by the United States Food and Drug Administration (FDA) to treat hospitalised coronavirus patients.





















.png)












No hay comentarios:
Publicar un comentario