
PSMA PET-CT Accurately Detects Prostate Cancer Spread, Trial Shows
, by NCI Staff
For some men with prostate cancer, results from a large clinical trial suggest that there may be a more effective imaging approach to detect the spread of their cancer to other parts of the body than the approach that is most commonly used.
Conducted in Australia, the trial included men diagnosed with localized prostate cancer that was thought to be at high risk of spreading beyond the prostate. In the study, an imaging method known as PSMA PET-CT was substantially more likely to detect metastatic tumors in these men than the standard imaging approach used in many countries, which involves a CT scan and a bone scan.
Use of PSMA PET-CT also was more likely than the standard approach to change the strategy doctors used to treat the cancer, the trial’s lead investigator, Michael Hofman, a professor of Nuclear Medicine at the Peter MacCallum Cancer Centre in Melbourne, and his colleagues reported March 22 in The Lancet.
The trial was not designed to show whether using PSMA PET-CT improved clinical outcomes like how long patients lived. But the results, Dr. Hofman said, build on evidence from other studies and current clinical practice in countries like Australia and Germany that suggest PSMA PET-CT is more likely to detect metastases than the conventional approach.
“For a diagnostic test like this, accuracy is the most important thing. You want an accurate test,” Dr. Hofman said. Because, in this patient population, he noted, the presence of metastases should affect how men are treated.
If the cancer has spread beyond the prostate at the time of diagnosis, treating the primary tumor in the prostate with surgery or radiation on their own “is a futile exercise,” Dr. Hofman said. Knowing early after diagnosis whether the cancer has already spread “is key to better optimizing the treatment for these men.”
The Food and Drug Administration (FDA) hasn’t approved any PSMA-targeted imaging agents, so PSMA PET-CT isn’t available in the United States outside of clinical studies, explained Lalitha Shankar, M.D., Ph.D., of the Cancer Imaging Program in NCI’s Division of Cancer Treatment and Diagnosis.
Several PSMA-targeted imaging agents are being studied, Dr. Shankar said, and “they are all quite promising.” But further studies are still needed, she continued. In particular, additional clinical trials that carefully examine how these imaging approaches “affect patient management” would be helpful, she said. “We also need to see whether there is an impact on outcomes.”
A Different Way to Detect Metastases
Most men diagnosed with prostate cancer have localized disease, meaning the cancer appears to be confined to the prostate gland. However, certain factors have been linked to a higher risk of the cancer eventually spreading (or having already spread).
Currently, in the United States and many other countries, most men diagnosed with high-risk localized prostate cancer undergo additional testing to see if there is evidence of metastatic cancer. For many years, that has been done with a conventional CT scan (which uses a form of x-rays) and a bone scan (a type of nuclear imaging test), the latter because prostate cancer often spreads to the bones.
But both imaging technologies have limitations. Neither is particularly good at finding individual prostate cancer cells, and thus can miss very small tumors. And bone scans can detect bone damage or abnormalities that were caused by something other than cancer (e.g., arthritis), resulting in “false-positive” findings that can lead to unnecessary additional testing.
So, researchers have been developing and testing other imaging agents that can find prostate cancer cells specifically in the body, Dr. Shankar explained.
As their name implies, PET-CT scans combine a CT scan with a PET scan, another type of nuclear imaging test that requires patients to receive intravenous injections of a radioactive “tracer” that can be detected on the scan.
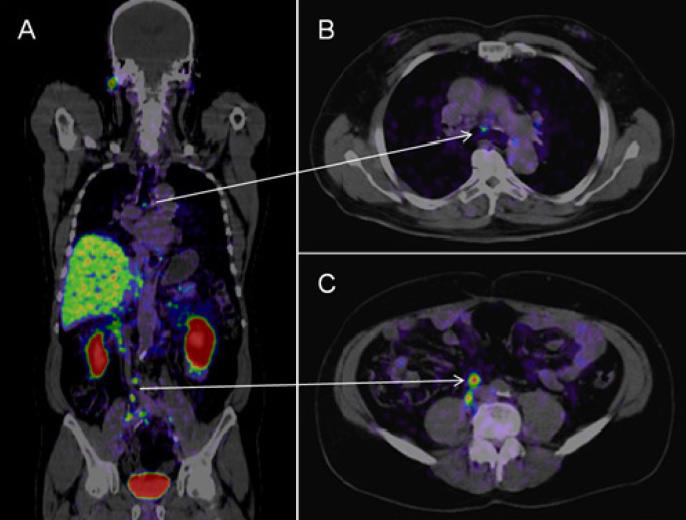
In a PSMA PET-CT, the tracer used for the PET scan includes a molecule that specifically binds to the PSMA protein, which is often found in large amounts on prostate cancer cells. That molecule is linked to a radioactive compound, or radioisotope. The radioisotope used in the Australian trial is called gallium-68 (Ga-68).
PSMA-targeted tracers that use other radioisotopes are also being widely studied, explained Martin Pomper, M.D., Ph.D., director of Nuclear Medicine and Molecular Imaging at the Johns Hopkins School of Medicine. Smaller studies have strongly suggested that PSMA PET-CT is better at detecting metastases in men with localized prostate cancer, Dr. Pomper said, so more definitive answers from larger studies have been anxiously awaited.
Greater Accuracy and Changing Treatment
Approximately 300 men were enrolled in the Australian trial, all with newly diagnosed localized prostate cancer (based on a prostate biopsy), and all were considered to have high-risk disease. For all men in the trial, the planned treatment was either surgery (prostatectomy) or radiation therapy to the prostate only.
Half the men were randomly assigned to initially undergo a CT and bone scan, and the other half to PSMA PET-CT.
Based on the imaging, PSMA PET-CT was 27% more accurate than the standard approach at detecting any metastases (92% versus 65%). Accuracy was determined by combining the scans’ sensitivity and specificity, measures that show a test’s ability to correctly identify when disease is present and not present.
PSMA PET-CT was more accurate for both metastases found in lymph nodes in the pelvis and in more distant parts of the body, including bone. Radiation exposure was also substantially lower with PSMA PET-CT than with the conventional approach.
The trial investigators also tracked how imaging results influenced clinicians’ treatment choices. Based on imaging findings, the initial treatment plan was changed for 15% of men who underwent conventional imaging compared with 28% of men who underwent PSMA PET-CT.
Another key finding, Dr. Hofman noted, was that PSMA PET-CT was much less likely to produce inconclusive, or equivocal, results (7% versus 23%).
That’s important, he continued, “because if you have a scan with equivocal findings, it often leads to more scans or biopsies or other tests.”
The Future of PSMA PET-CT
“This is a solid study and reflects the real-world experience” with PSMA PET-CT in other countries, Dr. Pomper said. Because there are several PSMA-targeted tracers, a next step will be to have them approved for use in the United States outside of clinical trials, he added.
He predicted that, eventually, the different PSMA tracers will be tested head to head.
The Australian trial adds to a growing body of research on improving the detection of metastatic tumors in men with prostate cancer. One imaging agent, fluciclovine F18 (Axumin)—which targets prostate cancer cells in a different way than PSMA-targeted tracers—is already approved in the United States for use in men with previously treated prostate cancer that appears to be progressing (based on rising PSA levels).
PSMA PET-CT is also being studied in this group of men, Dr. Shankar said. One small clinical trial that directly compared PSMA PET-CT with fluciclovine F18 PET-CT showed that the PSMA-targeted scan found more metastatic tumors, regardless of their location. NCI is funding a similar but larger clinical trial.
Dr. Pomper noted that PSMA also is found at relatively high levels in the vasculature of a number of other cancers—including kidney, thyroid, and breast—so he’s hopeful that PSMA PET-CT might be useful beyond prostate cancer.
Researchers at UCLA and the University of California San Francisco (UCSF) have filed separate applications with FDA for approval of a Ga-68 PSMA-radiotracer, according to Jeremie Calais, M.D., of the Jonsson Comprehensive Cancer Center at UCLA. Both applications could be approved by the end of 2020, Dr. Calais said.
However, the respective applications waive any exclusive rights to those tracers, he explained. Once FDA approves those applications, other institutions that have the manufacturing capabilities could produce their own tracers, using the same specifications as the UCLA and UCSF agents, and then undergo an abbreviated approval process with FDA to begin using them, he said.
In the meantime, it’s still unclear how PSMA PET-CT will affect clinical practice in the United States. But it already has in Australia and several European countries, Dr. Hofman said. In Australia, hospitals don’t need regulatory approval to manufacture their own PSMA-based tracers to use in patient care, so at least 60 hospitals in Australia routinely use PSMA PET-CT.
“Urologists and radiation oncologists in many places [in Australia] are already ordering this scan as the standard of care,” he said.
Concerns have also been raised about the costs associated with a broader use of PET-CT. Although costs can vary, in many countries PET-CT is currently more expensive than a CT scan and bone scan. However, if PSMA-targeted PET-CT begins to be more widely used, Dr. Pomper said he’s hopeful that the cost could come down through economies of scale, as has happened for FDG PET-CT, which is the most common form of PET-CT used in cancer.





















.png)












No hay comentarios:
Publicar un comentario