A new DRUG TRIALS SNAPSHOT is now available.

Drug Trials Snapshot: TUKYSA
TUKYSA is a drug for treatment of adults with human epidermal growth factor receptor (HER)2-positive breast cancer that has spread to other parts of the body including the brain (metastatic) or cannot not be removed by surgery.
It should be used in patients who have been previously treated for their metastatic disease with at least one anti-HER2 regimen and in combination with two other medications for the treatment of metastatic breast cancer (trastuzumab and capecitabine).
TUKYSA is a tablet taken by mouth two times a day in combination with trastuzumab and capecitabine.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
TUKYSA (tucatinib)
(too kye' sah)
Seattle Genetics
Approval date: April 17, 2020
(too kye' sah)
Seattle Genetics
Approval date: April 17, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TUKYSA is a drug for treatment of adults with human epidermal growth factor receptor (HER)2-positive breast cancer that has spread to other parts of the body including the brain (metastatic) or cannot not be removed by surgery.
It should be used in patients who have been previously treated for their metastatic disease with at least one anti-HER2 regimen and in combination with two other medications for the treatment of metastatic breast cancer (trastuzumab and capecitabine).
How is this drug used?
TUKYSA is a tablet taken by mouth two times a day in combination with trastuzumab and capecitabine.
What are the benefits of this drug?
The trial measured the length of time tumors did not grow after treatment (progression-free survival or PFS). The progression-free survival for patients taking TUKYSA together with trastuzumab and capecitabine was about 8 months compared to 6 months for patients taking a placebo with trastuzumab and capecitabine.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
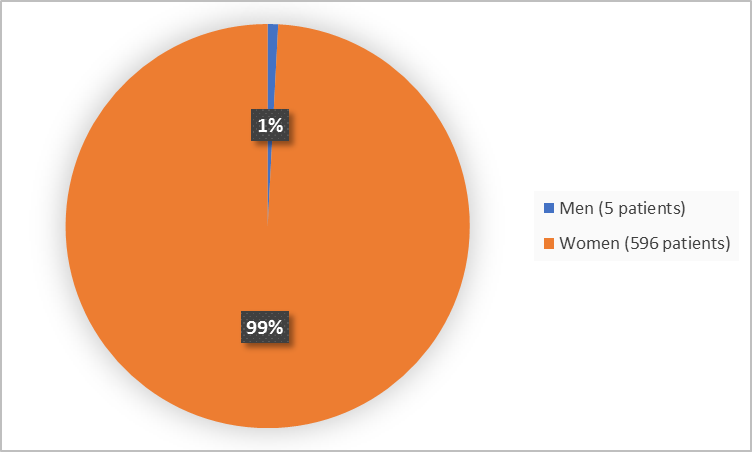
- Sex: Almost all included patients were women, therefore sex differences could not be determined.
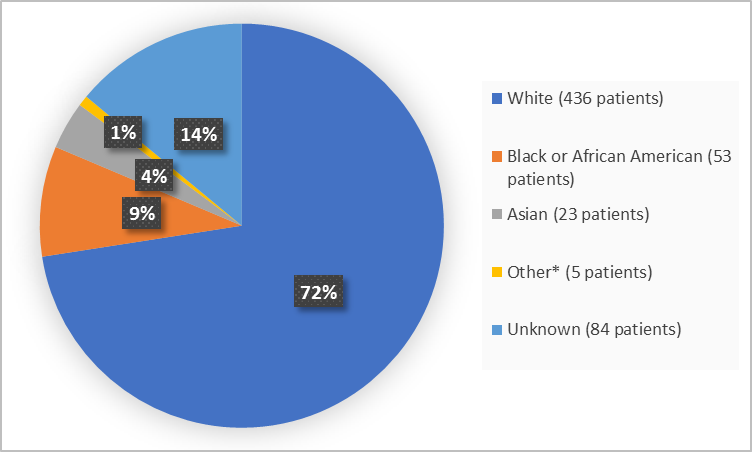
- Race: The majority of patients were White, therefore race differences could not be determined.
- Age: TUKYSA worked similarly in patients above and below 65 years of age.
What are the possible side effects?
TUKYSA may cause serious side effects including severe diarrhea, liver damage, and harm to an unborn baby.
The most common side effects of TUKYSA are diarrhea, palmar-plantar erythrodysesthesia (rash, redness, pain, swelling or blisters on the palms of the hands or soles of the feet) nausea, tiredness, liver toxicity, vomiting and mouth sores.
Were there any differences in side effects among sex, race and age?
- Sex: Almost all included patients were women therefore sex differences could not be determined.
- Race: The majority of patients were White, therefore race differences in side effects could not be determined.
- Age: The occurrence of overall side effects was similar in patients above and below 65 years of age. The occurrence of serious side effects1 was higher in patients older than 65 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved TUKYSA based on evidence from one clinical trial (NCT02614794) of patients with HER2-positive metastatic breast cancer. The trial was conducted at 157 sites in the United States, Canada, Europe, Israel and Australia.
Presented below is safety population (601 patients) which includes all patients from the trail who received at least one dose of TUKYSA and provided data for assessment of side effects.
Figure 1 summarizes by sex how many patients were in the clinical trial.
Figure 1. Baseline Demographics by Sex (safety population)
Adapted from FDA Review
Figure 2 summarizes patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race (safety population)
*includes Native Hawaiian or Other Pacific Islander and Multiple
Adapted from FDA Review
Figure 3 summarizes patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age (safety population)
Adapted from FDA Review
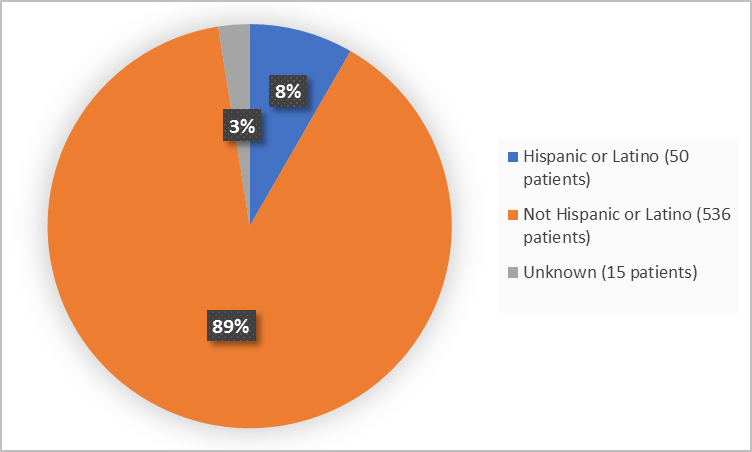
Figure 4 summarizes patients by ethnicity in the clinical trial.
Figure 4. Baseline Demographics by Ethnicity (safety population)
Adapted from FDA Review
How were the trials designed?
There was one trial that provided data for TUKYSA approval. The trial enrolled adult patients with HER2-positive metastatic breast cancer who have been treated presviously for their metastatic disease.
Patients received TUKYSA or placebo twice daily, in combination with trastuzumab and capecitabine. The treatment continued until the disease progressed or the side effects became too toxic.
The trial measured the length of time tumors did not grow (progression-free survival).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.






















.png)












No hay comentarios:
Publicar un comentario