
Volume 25, Number 10—October 2019
CME ACTIVITY - Synopsis
Edwardsiella tarda Bacteremia, Okayama, Japan, 2005–2016
Figures
Downloads
Article Metrics
Introduction

Medscape CME ACTIVITY
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at ; and (4) view/print certificate.
Release date: September 13, 2019; Expiration date: September 13, 2020
Learning Objectives
Upon completion of this activity, participants will be able to:
• Describe the clinical epidemiology and characteristics of Edwardsiella tarda bacteremia, according to a clinical series from Japan
• Determine treatment and outcomes of E. tarda bacteremia, according to a clinical series from Japan
• Identify seasonal distribution and other clinical implications of findings of this clinical series from Japan
CME Editor
Karen L. Foster, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Karen L. Foster has disclosed no relevant financial relationships.
CME Author
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC. Disclosures: Laurie Barclay, MD, has disclosed no relevant financial relationships.
Authors
Disclosures: Shinya Kamiyama, MD; Akira Kuriyama, MD, MPH; and Toru Hashimoto, MD, PhD, have disclosed no relevant financial relationships.
Abstract
Edwardsiella tarda is primarily associated with gastrointestinal disease, but an increasing number of cases involving extraintestinal disease, especially E. tarda bacteremia, have been reported. Using clinical information of E. tarda bacteremia patients identified during January 2005–December 2016 in Japan, we characterized the clinical epidemiology of E. tarda bacteremia. A total of 182,668 sets of blood cultures were obtained during the study period; 40 (0.02%) sets from 26 patients were positive for E. tarda. The most common clinical manifestations were hepatobiliary infection, including cholangitis, liver abscess, and cholecystitis. Overall 30-day mortality for E. tarda bacteremia was 12%, and overall 90-day mortality was 27%. The incidence of E. tarda infection did not vary by season. We more frequently observed hepatobiliary infection in patients with E. tarda bacteremia than in patients with nonbacteremic E. tarda infections. E. tarda bacteremia is a rare entity that is not associated with high rates of death.
Edwardsiella tarda, a gram-negative, facultative anaerobe that is a member of the family Enterobacteriaceae, typically is isolated from water environments and animals that inhabit water. It is primarily associated with gastrointestinal disease, but the number of reports of extraintestinal disease, such as septicemia, meningitis, cholecystitis, and osteomyelitis, has increased (1). However, little is known about the clinical epidemiology of E. tarda bacteremia. Therefore, we aimed to document the clinical epidemiology of E. tarda bacteremia, including common sources of infection, antimicrobial susceptibility, and seasonal distribution.
We retrospectively reviewed electronic medical records and clinical microbiology records in Kurashiki Central Hospital (Okayama, Japan), a 1,166-bed, tertiary-care hospital that provides care to ≈300,000 persons annually. Clinical specimens submitted to the microbiology laboratory included blood, sputum, urine, bile, ascites, feces, placenta, tissue, and pus. Information about identified bacteria and antimicrobial susceptibility were kept as microbiology laboratory records for each specimen. We considered bacteremia to exist when >1 set of blood cultures was positive. We identified all cultures growing E. tarda from clinical specimens submitted during January 2005–December 2016.
We processed blood culture samples using the BacT/Alert system (Sysmex bioMérieux Co. Ltd., ) and conducted microbial culture using KBM Chocolate HB Agar (Kohjin Bio Co. Ltd., ), KBM Sheep Blood Agar (Kohjin Bio Co. Ltd.), and BTB agar (Kyokuto Pharmaceutical Co. Ltd., ). We used different bacterial identification and antimicrobial susceptibility testing methods in our hospital throughout the study period. We used ID test EB-20 Nissui (Nissui Pharmaceutical Co. Ltd., ) for bacterial identification and Kirby–Bauer disk (Eiken Chemical Co. Ltd., ) for antimicrobial susceptibility testing from January 2005 through June 2007. EB-20 is a system to identify glucose-fermenting gram-negative rods by 20 patterns of biochemical properties, using hydrogen sulfide, indole, lysine, ONPG (2-nitrophenyl-β-D-galactopyranoside), adunit, inositol, rhamnose, mannit, esculin, Voges-Proskauer, arginine, urea, inositol, sorbitol, arabinose, phenylpyruvic acid, citric acid, ornithine, malonic acid, raffinose, and sugar. Thereafter, automatic systems were introduced at our hospital: DPS192 (Eiken Chemical Co. Ltd, ) during July 2007–February 2013 and MicroScan WalkAway (Beckman Coulter, Inc, ) during March 2013–March 2014. Since April 2014, we have used MALDI Biotyper (Bruker Daltonics GmbH, ), using the manufacturer-provided database, for bacterial identification. We judged the drug susceptibility of a microorganism based on clinical breakpoints set by the Clinical and Laboratory Standards Institute; in particular, we used the document M100-S22 (2) during June 1, 2013–December 31, 2016.
We collected all clinical information of patients with positive E. tarda bacteremia results from electronic medical records, including age, sex, underlying diseases, source of infection, antimicrobial drug administered, treatment period, and outcome. We defined chronic kidney disease as a serum creatinine level of >2.0 mg/dL (reference range 0.65–1.07 mg/dL) and chronic liver disease as liver cirrhosis or chronic hepatitis B or C infection. We defined nosocomial bloodstream infection, healthcare-associated bloodstream infection, community-acquired bloodstream infection, and febrile neutropenia according to the previous study and guideline (3,4). We defined 30-day mortality as patient death within 30 days after the onset of E. tarda bacteremia and 90-day mortality as patient death within 90 days after onset. We also collected information of patients with E. tarda nonbacteremic infections.
We described the clinical characteristics and 30-day mortality of patients with E. tarda bacteremia, along with the source of infection and antimicrobial susceptibility. We then compared the characteristics of patients with E. tarda bacteremia by 30-day mortality. We also compared the characteristics of patients with bacteremic and nonbacteremic E. tarda infections. We also conducted an exploratory multivariable logistic regression analysis to investigate the risk for E. tarda bacteremia incidence among all E. tarda infections.
Because a previous literature review suggested seasonal variation in the occurrence of E. tarda bacteremia (5), we thus examined whether such variation or trend existed in the cases in our study by using Cochran-Armitage test. We tested dichotomous variables with Fisher exact test and and continuous variables by Wilcoxon signed-rank test. Statistical analysis was performed using Stata version 15.1 (StataCorp, ). We considered p<0.05 to be statistically significant.
The Ethics Committee of Kurashiki Central Hospital approved this study (no. 2,527). Only persons with appropriate authorization had access to participants’ records, and patient confidentiality was maintained. Given the nature of a retrospective chart review, written consent from the patients was waived.
We obtained 182,668 sets of blood cultures during the study period, of which 19,234 sets were positive for some organisms and 40 sets from 26 patients were E. tarda–positive. E. tarda bacteremia was diagnosed in 26 patients (13 men and 13 women); their median age was 75 years (range 45–101 years) (Table 1).
Clinical Characteristics
Some patients had >1 underlying disease: solid tumors (12 patients), cardiovascular diseases (4 patients), diabetes mellitus (3 patients), gallstone disease (3 patients), chronic liver disease (2 patients), cerebrovascular disease (2 patients), and hematologic malignancy (1 patient) (Table 1). Four patients had no underlying disease. Sites of solid tumors included pancreas (3 patients), gallbladder/bile duct (3 patients), colon (2 patients), and esophagus, gastric, liver, and thyroid (1 patient each). Of the 12 patients with solid tumors, 4 were receiving chemotherapy for their cancer when E. tarda bacteremia occurred.
Clinical diagnoses by the site of infection were cholangitis (9 patients); liver abscess (6 patients); enterocolitis (4 patients); cholecystitis (3 patients); and spontaneous bacterial peritonitis, mycotic aneurysm, necrotizing fasciitis, empyema, osteomyelitis, and secondary peritonitis (1 patient each) (Table 2). Seventeen patients had community-acquired bloodstream infections. The source of infection was not identified in 5 patients, including 1 with febrile neutropenia; 3 patients had nosocomial bloodstream infections, and 6 had healthcare-associated bloodstream infections.
Patients with E. tarda bacteremia were older and more likely to have solid tumors than were patients with E. tarda nonbacteremic infections (Table 3). In addition, we observed hepatobiliary infection, such as cholangitis and liver abscess, more frequently in patients with bacteremia.
Because the cohort included 26 E. tarda bacteremia patients, we conducted a multivariable logistic regression analysis adjusted with 2 explanatory variables. We hypothesized that underlying liver disease and old age could be associated with the incidence of E. tarda bacteremia and selected these 2 variables as the covariates. Our analysis suggested that age >65 years was significantly associated with an increased risk for E. tarda bacteremia incidence (odds ratio 2.70; 95% CI 1.11–6.55; p = 0.028). However, underlying chronic liver disease was not the risk factor for E. tarda bacteremia (odds ratio 2.48; 95% CI 0.41–14.99; p = 0.32).
Treatment and Outcomes
All E. tarda strains isolated from blood cultures were susceptible to all tested antimicrobial drugs. E. tarda bacteremia patients were treated with a variety of antimicrobial drugs according to the treating physicians’ discretion (Table 3). The median duration of treatment was 12 days (range 1–77 days). Overall 30-day mortality for E. tarda bacteremia was 12% (3/26) and overall 90-day mortality 27% (7/26).
Patient 4 had end-stage hepatocellular carcinoma and liver failure. On day 2 after admission, E. tarda bacteremia developed; the source of infection was unidentified. He was treated with cefepime and promptly became afebrile. E. tarda bacteremia was considered controlled by cefepime; however, the patient died of hepatic failure on day 11.
In patient 20, necrotizing fasciitis was diagnosed, and E. tarda was detected from wound and blood cultures. Although meropenem and clindamycin were administered, he died on day 2.
Patient 26, who had end-stage alcoholic liver cirrhosis, was admitted for massive pleural effusion and ascites. E. tarda was detected from pleural effusion but not from ascites. Empyema and spontaneous bacterial peritonitis caused by E. tarda were diagnosed. Although these fluids were drained and antimicrobial drugs were given, she died on day 5.
Patient 5 was admitted for evaluation of fever and back pain. Blood cultures drawn on admission day revealed E. tarda, and he was treated with imipenem–cilastatin. However, his fever persisted. Computed tomography scan of the chest and abdomen revealed mycotic thoracic aneurysm, liver abscess, and vertebral osteomyelitis. He was treated with multiple antimicrobial drugs but died of a ruptured mycotic aneurysm on day 39.
In patients 6, 9, and 21, E. tarda bacteremia developed and improved with antimicrobial therapy. However, these patients died of underlying diseases.
Seasonal Variation in E. tarda Bacteremia
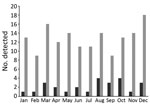
The incidence of E. tarda infection did not vary by season (Figure). We found no trend of E. tarda bacteremia incidence among all E. tarda infections when we examined them by month (p = 0.46) or by season, defined as a set of 3 months (p = 0.53).
E. tarda is associated with freshwater and marine life, including fish, reptiles, and amphibians (1). The organism resembles Salmonella biochemically and clinically (1). Salmonella usually ferments D-mannitol, urease, oxidase, and D-sorbitol, whereas E. tarda produces hydrogen sulfide and indole (6).
E. tarda is a rare human pathogen and is primarily associated with gastrointestinal diseases, including the asymptomatic carrier state (1). Approximately 80% of infections are intestinal. E. tarda causes a Salmonella-like gastrointestinal infection, usually self-limited enteritis, with intermittent watery diarrhea and low-grade fever (1,7).
The pathogenesis of E. tarda and its disease-causing mechanism remain unclear. Twelve classes of bacterial protein secretion systems are known; these systems transport virulence proteins into the cell and, in some cases, directly into the cytoplasm of a target cell (8). The bacterial type III and type VI secretion systems (T3SS and T6SS) are believed to play an essential role in E. tarda survival, replication, and virulence inside the host. In particular, T6SS is proposed to enable E. tarda to establish inside the host, cause severe systemic infection, and eventually kill the host.
We reviewed 26 cases of E. tarda bacteremia. Clinical diagnoses included 15 (58%) biliary tract infections (cholangitis, cholecystitis, and liver abscess). Eight of these patients had hepatobiliary diseases including cholangiocarcinoma, gallbladder cancer, pancreatic cancer, gallstone disease. Therefore, hepatobiliary diseases may be a predisposing factor of E. tarda biliary tract infections. However, our multivariable logistic regression found that only age >65 years was associated with the incidence of E. tarda bacteremia. We acknowledge that the sample size of our study and the number of E. tarda bacteremia incidence were still small, and thus the finding from our multivariable analysis might be only exploratory.
Previous studies reported high rates of death for E. tarda bacteremia, ranging from 22.7% to 44.6% (1,5,9). In contrast, the death rate for patients with E. tarda bacteremia in the cohort reported here was low at 12%. However, 2 of these 3 patients had end-stage liver disease; only 1 death among these patients was attributed to E. tarda bacteremia.
E. tarda is susceptible to most antimicrobial drugs, including tetracyclines, aminoglycosides, quinolones, antifolates, chloramphenicol, nitrofurantoin, fosfomycin, and most β-lactams (10), and is naturally resistant to benzylpenicillin, colistin, and polymyxin B (1,11). In our study, E. tarda was susceptible to most commonly used antimicrobial drugs. E. tarda susceptibilities to colistin and polymyxin B are unknown because susceptibility testing is not routinely performed for these drugs in our institution. Previous studies have shown that all strains of E. tarda were positive for β-lactamase production examined with nitrocefin β-lactamase disks, but an ampicillin-resistant E. tarda strain has not been reported (10,11). Whether E. tarda isolates detected in our institution produced β-lactamase is not clear because we did not perform the β-lactamase test, but 5 cases were successfully treated with ampicillin.
Hirai et al. suggested that E. tarda bacteremia is likely to develop during summer and autumn months in the Northern Hemisphere (8). The authors conducted a literature review of 77 E. tarda bacteremia cases reported from diverse areas and suggested seasonal variation in incidence for 22 cases. Our study of 26 E. tarda bacteremia cases suggests no such seasonal distribution. Several possible reasons might account for this discrepancy. First, E. tarda can colonize. In our study, hepatobiliary infection (such as cholangitis, cholecystitis, and liver abscess) was diagnosed in 58% (15/26) patients, and patients colonizing E. tarda developed E. tarda bacteremia. Second, diversity might exist in the patients’ dietary patterns. E. tarda frequently infects fish. Hirai et al. included patients from many parts of the world, so the intake of fish might have differed according to the season or geographic area across reports. In contrast, our study included only people in a single area of Japan who habitually ate raw seafood, such as sashimi, throughout the year; this tendency might have led to no seasonal variation of E. tarda bacteremia incidence. Third, our study had no missing clinical data for any patients, whereas Hirai et al. examined 22 of all 77 eligible patients, which might have rendered their analysis vulnerable to information bias.
Our study had some strengths. First, we elucidated that no seasonal variation existed in E. tarda bacteremia in this population. Second, we described the characteristics of each patient with E. tarda bacteremia and provided risk factors for E. tarda bacteremia incidence among all E. tarda infections.
Our study also had some limitations. First, the number of blood cultures submitted increased in recent years in our hospital. The number of blood cultures submitted in 2016 nearly doubled that for 2005. This increase might have resulted in the underestimation of E. tarda bacteremia in the earlier years of our study period. Second, ours was a retrospective and single-center study. However, our study had no missing data regarding clinical information. Furthermore, we successfully presented a particularly large case series of E. tarda bacteremia.
In conclusion, E. tarda bacteremia is a rare disease that is not associated with high rates of death. E. tarda bacteremia patients in our cohort in Japan had more severe underlying diseases, such as hepatobiliary disease and solid tumors, than did patients in previous studies. Hepatobiliary infections, such as cholangitis, cholecystitis, and liver abscess, are the most common clinical manifestations in patients with E. tarda bacteremia. The major underlying diseases in this study were hepatobiliary diseases and malignancy. Furthermore, E. tarda strains we isolated were susceptible to most antimicrobial drugs, including β-lactams, aminoglycoside, tetracycline, fosfomycin, fluoroquinolone, and trimethoprim/sulfamethoxazole, and E. tarda bacteremia was successfully treated with ampicillin. Finally, we observed no seasonal distribution of E. tarda bacteremia. Risk factors for E. tarda bacteremia–related death remain to be investigated.
Dr. Kamiyama is the medical director at Kurashiki Central Hospital. His primary research interests include cytomegalovirus infections in critically ill patients and transplant patients.
References
- Janda JM, Abbott SL. Infections associated with the genus Edwardsiella: the role of Edwardsiella tarda in human disease. Clin Infect Dis. 1993;17:742–8.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty-third informational supplement (M100-S23). Wayne (PA): The Institute; 2013.
- Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care—associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7.
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al.; Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52:e56–93.
- Hirai Y, Asahata-Tago S, Ainoda Y, Fujita T, Kikuchi K. Edwardsiella tarda bacteremia. A rare but fatal water- and foodborne infection: Review of the literature and clinical cases from a single centre. Can J Infect Dis Med Microbiol. 2015;26:313–8.
- Wilson JP, Waterer RR, Wofford JD Jr, Chapman SW. Serious infections with Edwardsiella tarda. A case report and review of the literature. Arch Intern Med. 1989;149:208–10.
- Schlenker C, Surawicz CM. Emerging infections of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2009;23:89–99.
- Green ER, Mecsas J. Bacterial secretion systems: an overview. Microbiol Spectr. 2016;4:4.
- Wang IK, Kuo HL, Chen YM, Lin CL, Chang HY, Chuang FR, et al. Extraintestinal manifestations of Edwardsiella tarda infection. Int J Clin Pract. 2005;59:917–21.
- Clark RB, Lister PD, Janda JM. In vitro susceptibilities of Edwardsiella tarda to 22 antibiotics and antibiotic-β-lactamase-inhibitor agents. Diagn Microbiol Infect Dis. 1991;14:173–5.
- Stock I, Wiedemann B. Natural antibiotic susceptibilities of Edwardsiella tarda, E. ictaluri, and E. hoshinae. Antimicrob Agents Chemother. 2001;45:2245–55.
Figure
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to . Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers.
You must be a registered user on . If you are not registered on , please click on the “Register” link on the right hand side of the website.
Only one answer is correct for each question. Once you successfully answer all post-test questions, you will be able to view and/or print your certificate. For questions regarding this activity, contact the accredited provider, . For technical assistance, contact . American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please go to . The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate, and present it to your national medical association for review.
Article Title:
Edwardsiella tarda Bacteremia, Okayama, Japan, 2005–2016
Edwardsiella tarda Bacteremia, Okayama, Japan, 2005–2016
CME Questions
1. Your patient is a 75-year-old man with a liver tumor who is suspected of having Edwardsiella tarda bacteremia (ETB). According to the clinical series of 26 patients with ETB by Kamiyama and colleagues, which of the following statements about the clinical epidemiology and characteristics of ETB is correct?
A. The most common clinical manifestation was urinary tract infection
B. Patients with ETB were older than patients with nonbacteremic E. tarda infection and had higher rates of hepatobiliary infections and solid tumors
C. The most common underlying disease was hematologic malignancy
D. More than half of the patients had hospital-acquired bloodstream infections
2. According to the clinical series of patients with ETB by Kamiyama and colleagues, which of the following statements about treatment and outcomes of ETB is correct?
A. E. tarda strains isolated from blood cultures were resistant to most tested antibiotics
B. More than half of patients in this cohort died within 90 days of developing ETB
C. E. tarda is naturally resistant to benzylpenicillin, colistin, and polymyxin B
D. ETB could not be successfully treated with ampicillin
3. According to the clinical series of patients with ETB by Kamiyama and colleagues, which of the following statements about seasonal distribution and other clinical implications is correct?
A. In this series, E. tarda infection more likely occurred during summer and autumn
B. This series showed that ETB is relatively common
C. Mortality of ETB in this series was higher than previously reported
D. E. tarda is a rare human pathogen causing salmonella-like gastrointestinal disease, usually self-limited enteritis, in »80% of infections
Original Publication Date: 9/12/2019





















.png)












No hay comentarios:
Publicar un comentario