A new DRUG TRIALS SNAPSHOT is now available

XENLETA is an antibacterial medicine used to treat adult patients with community-acquired bacterial pneumonia (CABP).
Pneumonia is a type of lung infection. Community-acquired bacterial pneumonia (CABP) develops in people with limited or no contact with hospitals or health care centers.
XENLETA can used in two different ways:
- It can be given by a healthcare professional as intravenous or IV infusion of 150 mg over 60 minutes twice a day for 5 to 7 days. Patients may switch to taking tablets to complete the treatment.
- It can be taken as a tablet (600mg) every 12 hours for 5 days.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov
XENLETA (lefamulin)
(zen-LET-uh)
NABRIVA Therapeutics, Inc.
Approval date: August 19, 2019
(zen-LET-uh)
NABRIVA Therapeutics, Inc.
Approval date: August 19, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
XENLETA is an antibacterial medicine used to treat adult patients with community-acquired bacterial pneumonia (CABP).
Pneumonia is a type of lung infection. Community-acquired bacterial pneumonia (CABP) develops in people with limited or no contact with hospitals or health care centers.
How is this drug used?
XENLETA can used in two different ways:
- It can be given by a healthcare professional as intravenous or IV infusion of 150 mg over 60 minutes twice a day for 5 to 7 days. Patients may switch to taking tablets to complete the treatment.
- It can be taken as a tablet (600mg) every 12 hours for 5 days.
What are the benefits of this drug?
XENLETA worked similarly to moxifloxacin (an antibacterial medication used to treat CABP) in improving signs and symptoms of pneumonia (cough, sputum production, chest pain, or difficulty breathing).
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
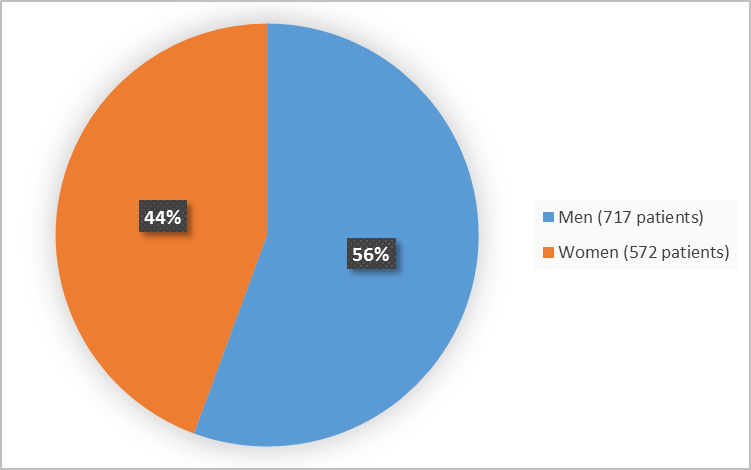
- Sex: XENLETA worked similarly in men and women.
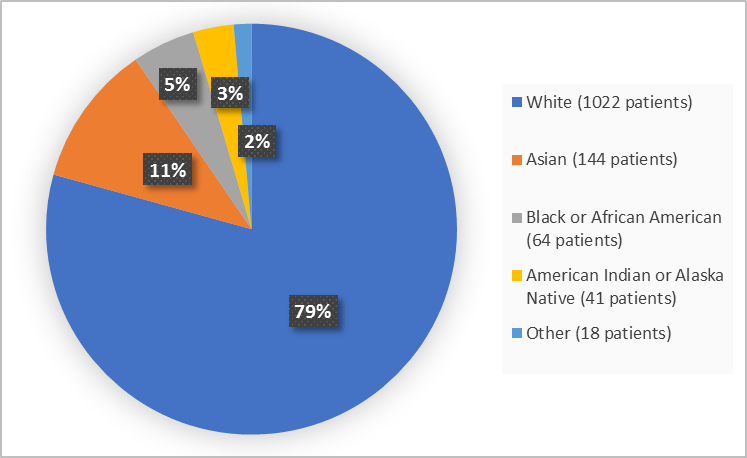
- Race: XENLETA worked similarly in White and Asian patients. Differences in how well the drug worked among other races could not be determined because of the small number of patients of other races.
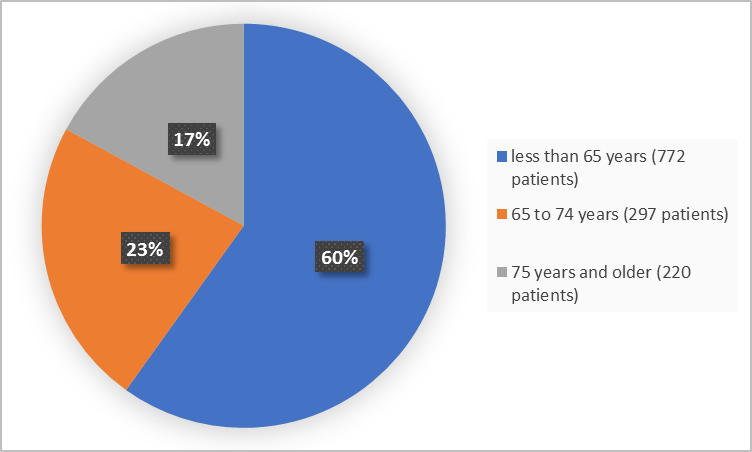
- Age: XENLETA worked similarly in patients younger than and older than 65 years of age.
What are the possible side effects?
XENLETA may cause serious side effects including changes in the heart rhythm, diarrhea caused by Clostridium difficile, and harm to an unborn baby.
The most common side effects are diarrhea, nausea, injection site reactions, liver enzyme elevation, and vomiting.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in all races.
- Age: The occurrence of side effects was similar between patients younger and older than 65 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved XENLETA based on evidence from two clinical trials (Trial 1/NCT02559310 and Trial 2/NCT02813694) of 1289 adult patients with CABP.
Trial 1 was conducted at 66 sites in Europe, Central and South America, Africa, Asia, and the United States. Trial 2 was conducted at 99 sites in Europe, Central and South America, Africa, Asia, and the United States.
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics by Race
Race | Number of Patients | Percentage of Patients |
|---|---|---|
White | 1022 | 79 |
Black or African American | 64 | 5 |
Asian | 144 | 11 |
American Indian or Alaska Native | 41 | 3 |
Other | 18 | 1 |
FDA Review
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
The benefit and side effects of XENLETA were evaluated in two clinical trials of adult patients 18 – 97 years of age with CABP. Across the 2 trials, 1289 adult patients with CABP were randomized to treatment (646 to XENLETA and 643 to comparator), out of which 641 patients were treated with XENLETA and 641 patients were treated with comparator.
Trial 1 compared 5 to 10 days of XENLETA therapy (IV infusion for at least 3 days with optional switch to tablet to complete 5 to 10 days of treatment) to 7 to 10 days of moxifloxacin ± linezolid therapy. Trial 2 compared 5 days of XENLETA tablet therapy to 7 days of moxifloxacin therapy. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed.
The benefit of XENLETA was assessed based on the response to treatment 72 to 120 hours after the first dose of treatment, comparing patients in the XENLETA and moxifloxacin groups. A positive response was defined as improvement from baseline on at least 2 out of 4 symptoms (cough, coughing up and spitting out phlegm, pain in the chest when breathing in or out, and difficulty breathing) with no worsening of other symptoms.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.





















.png)












No hay comentarios:
Publicar un comentario