A new DRUG TRIALS SNAPSHOT is now available.

WAKIX is a drug used to treat excessive daytime sleepiness in adults with narcolepsy.
Narcolepsy is a sleep disorder with excessive daytime sleepiness. Some patients with narcolepsy also experience sudden loss of muscle tone (cataplexy).
WAKIX is a tablet taken by mouth once every day in the morning upon wakening.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots: WAKIX
WAKIX (pitolisant)
way – kicks
Harmony Bioscience
Approval date: August 14, 2019
way – kicks
Harmony Bioscience
Approval date: August 14, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
WAKIX is a drug used to treat excessive daytime sleepiness in adults with narcolepsy.
Narcolepsy is a sleep disorder with excessive daytime sleepiness. Some patients with narcolepsy also experience sudden loss of muscle tone (cataplexy).
How is this drug used?
WAKIX is a tablet taken by mouth once every day in the morning upon wakening.
What are the benefits of this drug?
Patients who received WAKIX had less daytime sleepiness in comparison to patients who received placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: WAKIX worked similarly in men and women.
- Race: The majority of patients were White. The number of patients of other races was limited; therefore, differences in how well WAKIX worked among races could not be determined.
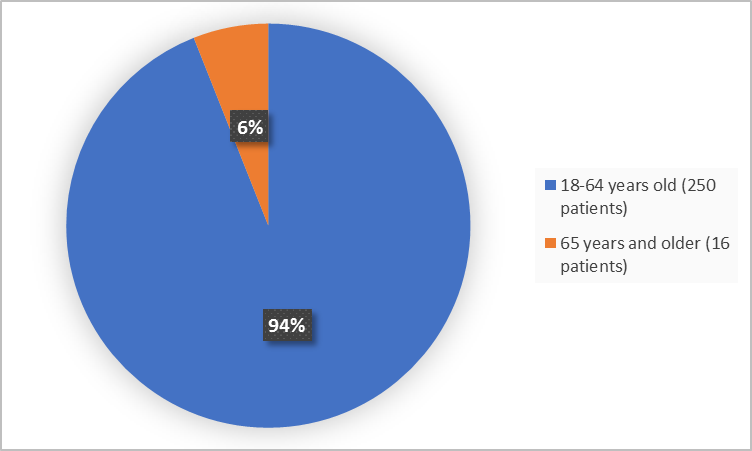
- Age: The majority of patients were adults less than 65 years of age. The number of patients older than 65 years of age was limited; therefore, differences in how well WAKIX worked between patients younger and older than 65 years of age could not be determined.
What are the possible side effects?
WAKIX may cause heart rhythm problems (because of change in heart electrical activity called QT prolongation), especially in those who have underlying heart rhythm problems or who take drugs that alter the heart rhythm.
The most common side effects of WAKIX are difficulty sleeping, nausea, and feeling worried.
Were there any differences in side effects among sex, race and age?
- Sex:The occurrence of side effects was similar between men and women.
- Race:The majority of patients were White. The number of patients of other races was limited; therefore, differences in side effects among races could not be determined
- Age:The majority of patients were adults less than 65 years of age. The number of patients older than 65 years was limited; therefore, differences in side effects between patients younger and older than 65 years of age could not be determined.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved WAKIX for excessive daytime sleepiness in patients with narcolepsy based primarily on evidence from two trials (Trial 1/NCT 01067222, Trial 2/NCT 01638403). An additional trial (Trial 3/NCT01800045), in which patients with a different type of narcolepsy were exposed to the same dose of WAKIX, was used to add data for evaluation of side effects. The trials were conducted in Europe and South America.
Demographics of the patients who provided data on side effects (safety population) are presented below. The population that provided data for benefits of WAKIX (efficacy population) is presented in Table 7, under the MORE INFO section.
Figure 1 below summarizes how many men and women were in the clinical trials used to evaluate safety.
Figure 1. Demographics by Sex (safety population)
FDA Review
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trials used to evaluate safety.
Figure 2. Demographics by Race (safety population)
*Other includes one American Indian or Alaskan Native; the data for other 115 patients are missing or were not collected.
FDA Review
Table 1. Demographics of Safety Trials by Race
Race | Number of Patients | Percentage of Patients |
White | 146 | 55% |
Black or African American | 4 | 1% |
American Indian or Alaskan Native | 1 | Less than 1 % |
Not Collected or Missing* | 115 | 43% |
*Some data not collected per European regulatory authority
FDA Review
Figure 3 summarizes the percentage of patients by age group in the clinical trials used to evaluate safety.
FDA Review
How were the trials designed?
The benefits and side effects of WAKIX were primarily evaluated in two clinical trials.
Both trials enrolled adult patients with narcolepsy and excessive daytime sleepiness. Patients received WAKIX, placebo, or an approved drug for narcolepsy for 8 weeks. For patients receiving WAKIX, the dose could be increased during the first 3 weeks but had to remain the same for the next 5 weeks. Neither the patients nor the healthcare providers knew which treatment was being given during the trial.
The benefit of WAKIX was evaluated by comparing changes in daytime sleepiness during the trial between WAKIX- and placebo-treated patients. To measure the daytime sleepiness, the investigators used a scale called the Epworth Sleepiness Scale (ESS). The ESS asks patients to rate the likelihood that they would fall asleep while doing eight daily activities (such as sitting and reading or watching television). Patients rate each item from 0 (would never doze) to 3 (high chance of dozing).
Data from one additional trial were used to evaluate the side effects of WAKIX. This trial enrolled adult patients with different type of narcolepsy for which they also received WAKIX.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.























.png)












No hay comentarios:
Publicar un comentario