
A new DRUG TRIALS SNAPSHOT is now available.
Drug Trials Snapshots: ROZLYTREK
ROZLYTREK is a drug used to treat adult patients with a type of non-small cell lung cancer (NSCLC) which:
- is caused by an abnormal ROS1 gene and,
- has spread to other parts of the body (metastatic).
ROZLYTREK is a capsule taken by mouth once a day.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
ROZLYTREK (entrectinib)
roz lye' trek
Genentech, Inc.
Approval date: August 15, 2019
roz lye' trek
Genentech, Inc.
Approval date: August 15, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ROZLYTREK is a drug used to treat adult patients with a type of non-small cell lung cancer (NSCLC) which:
- is caused by an abnormal ROS1 gene and,
- has spread to other parts of the body (metastatic).
How is this drug used?
ROZLYTREK is a capsule taken by mouth once a day.
What are the benefits of this drug?
Forty (78%) of 51 patients with NSCLC who received ROZLYTREK experienced complete or partial shrinkage of their tumors. Tumor shrinkage lasted more than 12 months for 55% % of those patients.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The difference in how well the drug worked in clinical trials among sex, race and age groups could not be determined because of the small sample sizes.
What are the possible side effects?
ROZLYTREK may cause serious side effects including congestive heart failure, nervous system problems, bone fractures, liver toxicity, increased uric acid in the blood, heart rhythm problems (because of changes in heart electrical activity called QT prolongation) and vision problems.
The most common side effects of ROZLYTREK are tiredness, constipation, taste changes, body swelling, dizziness, and diarrhea.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority patients in the clinical trial were White or Asian. Differences in side effects among races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in side effects between patients below and above 65 years of age could not be determined.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved ROZLYTREK based on the evidence from four clinical trials of patients with various types of solid tumors: Trial 1 (EudraCT 2012-000148-88), Trial 2 (NCT02097810), Trial 3 (NCT02568267), and Trial 4 (NCT02650401). The trials were conducted in the United States, Europe and Asia/Pacific region.
A subgroup of 51 patients with NSCLC from Trials 1, 2 and 3 that provided data characterizing the benefit of ROZLYTREK (efficacy population) is presented in Table 7 under MORE INFO.
All 355 patients (regardless of tumor types) from the four trials that provided data for the side effects of ROZLYTREK (safety population) are presented below.
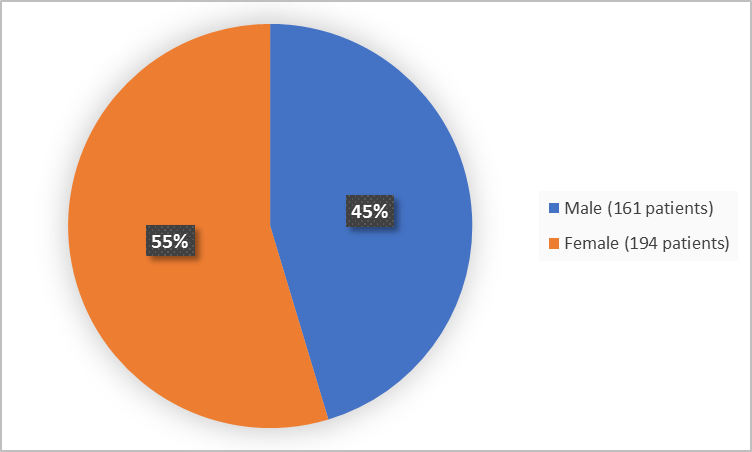
Figure 1 below summarizes how many patients were in the clinical trials by sex.
Figure 1. Baseline Demographics by Sex (safety population)
Clinical Trial Data
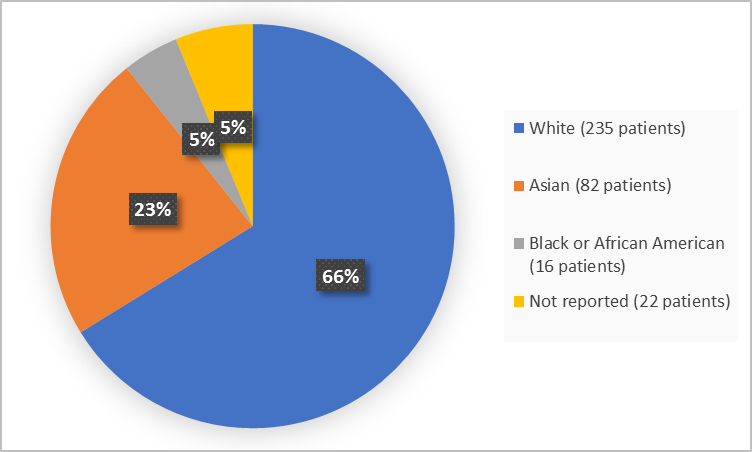
Figure 2 and Table 1 below summarize the percentage of patients in the clinical trials by race.
Figure 2. Baseline Demographics by Race (safety population)
Clinical Trial Data
Table 1. Demographics of Trial by Race (safety population)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 235 | 66 |
| Black or African American | 16 | 5 |
| Asian | 82 | 23 |
| Not Reported | 22 | 5 |
Clinical Trial Data
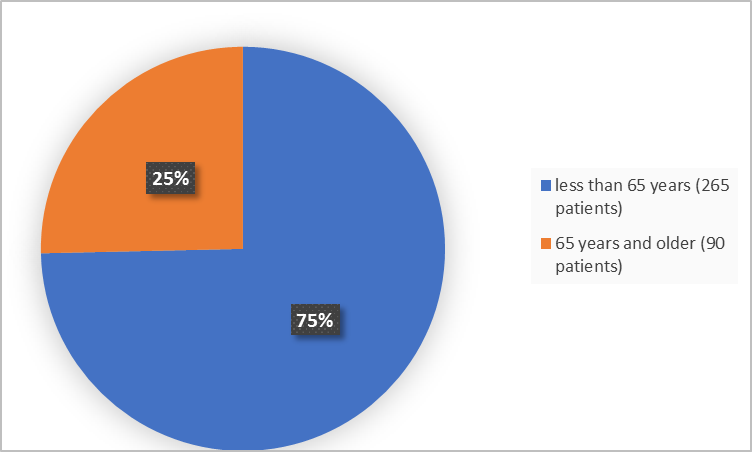
Figure 3 below summarizes the percentage of patients in the clinical trials by age.
Figure 3. Baseline Demographics by Age (safety population)
Clinical Trial Data
How were the trials designed?
The side effects of ROZLYTREK for NSCLC were evaluated in all patients enrolled in four clinical trials, and the benefit was evaluated in a subgroup of patients enrolled in three clinical trials.
Most adults received 600mg ROZLYTREK orally once a day until either tumor progression or intolerable side effects.
The benefit of ROZLYTREK for the treatment of NSCLC was evaluated by measuring the percentage of patients who achieved complete or partial shrinkage of their tumors (overall response rate) and by measuring the duration of that benefit (duration of response).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.





















.png)












No hay comentarios:
Publicar un comentario