A new DRUG TRIALS SNAPSHOT is now available.

RINVOQ is a drug used to treat adult patients with moderately to severely active rheumatoid arthritis (RA) in whom methotrexate [(MTX)a drug used to treat active arthritis] did not work well or could not be tolerated.
RINVOQ is a tablet that is taken once daily by mouth.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots: RINVOQ
RINVOQ (upadacitinib)
RIN-voke
AbbVie Inc
Approval date: August 16, 2019
RIN-voke
AbbVie Inc
Approval date: August 16, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
RINVOQ is a drug used to treat adult patients with moderately to severely active rheumatoid arthritis (RA) in whom methotrexate [(MTX)a drug used to treat active arthritis] did not work well or could not be tolerated.
How is this drug used?
RINVOQ is a tablet that is taken once daily by mouth.
What are the benefits of this drug?
In the clinical trials, a greater proportion of patients who received RINVOQ achieved an improvement in the signs and symptoms of RA in comparison to patients who received comparator drug or placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: RINVOQ worked similarly in men and women.
- Race: RINVOQ worked similarly in White, Black or African American, and Asian races.
- Age: RINVOQ worked similarly in all age groups tested (younger than 40 years of age, 40 to 64 years of age, and 65 years of age and older).
What are the possible side effects?
RINVOQ may cause serious side effects including;
- life threatening infections that may lead to hospitalization or death,
- increased risk of lymphoma (immune system cancer) and some other cancers and
- blood clots in the veins and arteries.
Other serious side effects include tears in the stomach or intestines, low blood cell counts, abnormal liver tests, harm to a fetus, and increased cholesterol.
The most common side effects are upper respiratory infections, nausea, cough, and fever.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar among races.
- Age: The occurrence of side effects increased with age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved RINVOQ based on evidence from five clinical trials (Trial 1/NCT02706873, Trial 2/NCT02706951, Trial 3/NCT02675426, Trial 4/NCT02629159, Trial 5/NCT02706847) of 3,141 patients with active rheumatoid arthritis (RA). The trials were conducted in Australia, New Zealand, Israel, South Africa, Asia, North/Central/South America, and Europe.
Demographics of the population that provided data for the assessment of side effects (safety population) are described in Table 8.
The figure below summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
FDA Review
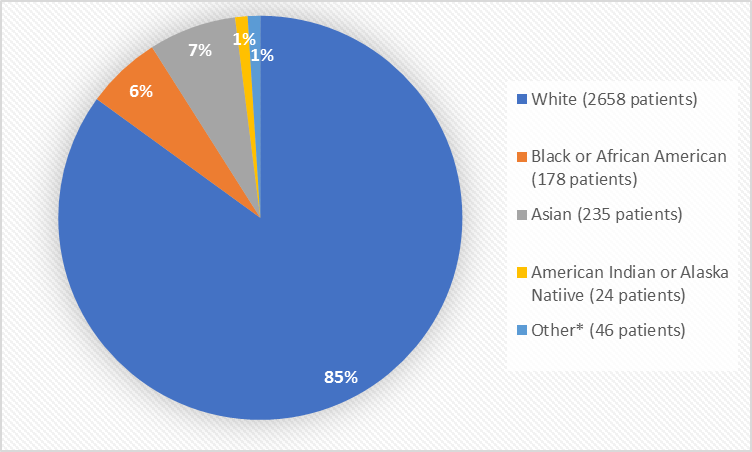
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
*Other includes Native Hawaiian or Other Pacific Islander
FDA Review
Table 1. Demographics of Efficacy Trials by Race
Race | Number of Patients | Percentage of Patients |
|---|---|---|
White | 2658 | 85% |
Black or African American | 178 | 6% |
Asian | 235 | 7% |
American Indian or Alaska Native | 24 | 1% |
Native Hawaiian or Other Pacific Islander | 6 | Less than 1% |
Other | 40 | 1% |
FDA Review
Figure 3 summarizes the percentage of patients by race in the clinical trials used to evaluate efficacy.
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
Five trials established the benefits and side effects of RINVOQ. Trials enrolled patients with moderate to severe active RA in whom disease-modifying antirheumatic drugs (DMARDS) did not work well or could not be tolerated. All patients had at least 6 tender and 6 swollen joints, and increased levels of high sensitivity C-reactive protein (hsCRP). hsCRP is a substance produced by the body to protect itself from illness. Trials lasted up to 5 years.
Trial 1 enrolled patients who had never been treated with MTX. Patients were randomly assigned to receive one of two doses of RINVOQ or MTX daily for 24 weeks. Neither the patient nor the healthcare providers knew which medication was being given until after this 24-week treatment period.
Trial 2 enrolled patients in whom MTX did not work well. Patients were randomly assigned to receive one of two doses of RINVOQ daily by mouth or continue their usual dose of MTX for 14 weeks. At Week 14, patients who were assigned to MTX received RINVOQ by mouth daily. Neither the patient nor the healthcare providers knew which medication was being given.
Trial 3 enrolled patients in whom DMARDS did not work well. Patients were randomly assigned to receive one of two doses of RINVOQ or placebo daily by mouth in addition to DMARDS for 12 weeks. At Week 12, patients who received placebo were re-assigned to RINVOQ daily. Neither the patient nor the healthcare providers knew which medication was being given.
Trial 4 enrolled patients in whom MTX did not work well. Patients were randomly assigned to receive RINVOQ or placebo daily by mouth in addition to MTX for 14 weeks. Patients receiving placebo who did not have adequate improvement of signs and/or symptoms could be switched to RINVOQ after Week 14. At Week 26, all patients receiving placebo were switched to RINVOQ once daily by mouth. Neither the patient nor the healthcare providers knew which medication was being given.
Trial 5 enrolled patients in whom DMARDS did not work well or could not be tolerated. Patients were randomly assigned to receive one of two doses of RINVOQ or placebo treatment daily added to DMARDs for 12 weeks. At Week 12, patients who received placebo were re-assigned to RINVOQ daily.
The benefit of RINVOQ was measured by comparing the proportion of patients treated with RINVOQ who achieved an American College of Rheumatology 20 (ACR20) response at Week 12 or Week 14 to the proportion of patients treated with MTX or placebo who achieved an ACR20 response. ACR20 is a 20% improvement in signs and symptoms of RA.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.























.png)












No hay comentarios:
Publicar un comentario