A new DRUG TRIALS SNAPSHOT is now available.

Drug Trials Snapshots: NOURIANZ
NOURIANZ is a drug used to treat “off” episodes in patients with Parkinson’s disease (PD). An “off” episode is a time when a patient’s medications are not working well, leading to an increase in Parkinson’s symptoms, such as tremor and difficulty walking.
NOURIANZ is a tablet taken by mouth once daily in addition to drugs for the treatment of Parkinson’s disease that contain levodopa/carbidopa combination.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov
NOURIANZ (istradefylline)
Nue’-ree-anz
Kyowa Kirin, Inc.
Approval date: August 27, 2019
Nue’-ree-anz
Kyowa Kirin, Inc.
Approval date: August 27, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
NOURIANZ is a drug used to treat “off” episodes in patients with Parkinson’s disease (PD). An “off” episode is a time when a patient’s medications are not working well, leading to an increase in Parkinson’s symptoms, such as tremor and difficulty walking.
How is this drug used?
NOURIANZ is a tablet taken by mouth once daily in addition to drugs for the treatment of Parkinson’s disease that contain levodopa/carbidopa combination.
What are the benefits of this drug?
Patients treated with NOURIANZ had decreased “off” time compared to patients treated with placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
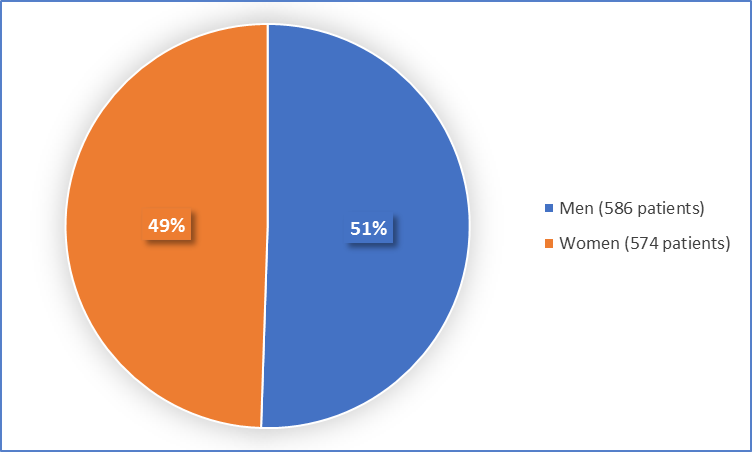
- Sex: NOURIANZ worked similarly in men and women.
- Race: Differences in how well the drug worked among races could not be determined.
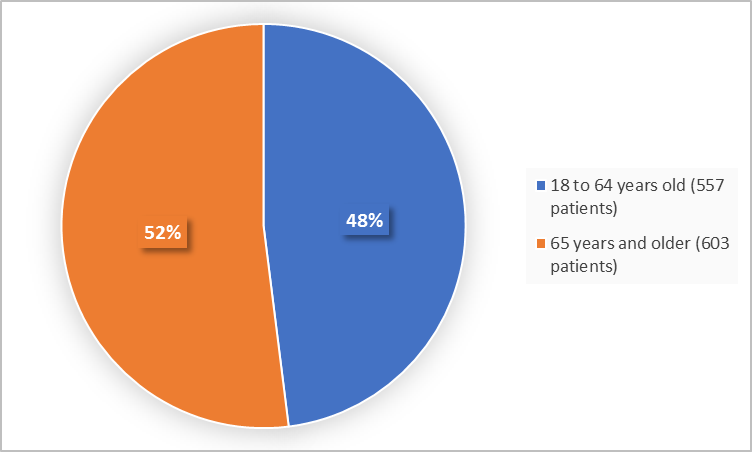
- Age: NOURIANZ worked similarly in patients younger and older than 65 years of age.
What are the possible side effects?
NOURIANZ can cause uncontrolled movement (dyskinesia), hallucinations, and unusual urges (impulse control or compulsive behaviors).
The most common side effects of NOURIANZ are uncontrolled movements, dizziness, constipation, nausea, hallucination, and insomnia.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects between men and women was similar.
- Race: The occurrence of side effects was similar between White and Asian patients.
- Age: The occurrence of side effects was similar between patients younger and older than 65 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved NOURIANZ based on evidence from 4 clinical trials (Trial 1/ NCT00456586, Trial 2/NCT00199407, Trial 3/NCT00455507, Trial 4/NCT00955526) of 1,160 patients with PD whose symptoms were not well controlled while receiving their regular PD treatment. Trial 1 was conducted in the US and Canada, Trial 2 in the US, and Trials 3 and 4 in Japan.
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Trials by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 400 | 34% |
| Black or African-American | 9 | 1% |
| Asian | 743 | 64% |
| Other | 8 | 1% |
FDA Review
Figure 3 summarizes the percentage of patients by age group in the trials.
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
There were four 12-week trials conducted in PD patients with inadequate control of their Parkinson’s symptom (“off” time) while receiving carbidopa/levodopa and other PD medications. Patients were randomly selected to receive either NOURIANZ or placebo pill once a day for 12 weeks. Neither the patients nor the health care providers knew which new treatment was being given until the trial was completed 12 weeks later.
In all trials, patients kept daily diaries of the number of hours they were awake and the number of hours of “off” time. The benefit was evaluated by measuring the change from baseline in total daily “off” time in NOURIANZ and placebo receiving patients.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.






















.png)












No hay comentarios:
Publicar un comentario