A new DRUG TRIALS SNAPSHOT is now available.

Drug Trials Snapshots: IBSRELA
IBSRELA is a treatment for adults with a disease of the gut called irritable bowel syndrome with constipation commonly referred to as IBS-C.
IBSRELA is a tablet taken by mouth two times each day.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov
IBSRELA (tenapanor)
(ibs rel`a)
Ardelyx
Approval date: September 12, 2019
(ibs rel`a)
Ardelyx
Approval date: September 12, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
IBSRELA is a treatment for adults with a disease of the gut called irritable bowel syndrome with constipation commonly referred to as IBS-C.
How is this drug used?
IBSRELA is a tablet taken by mouth two times each day.
What are the benefits of this drug?
Higher proportion of patients who received IBSRELA experienced a decrease in abdominal pain and more frequent bowel movements in comparison to patients who received placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The majority of patients in the trials were women. IBSRELA was similarly effective in men and women.
- Race: IBSRELA was similarly effective in different races.
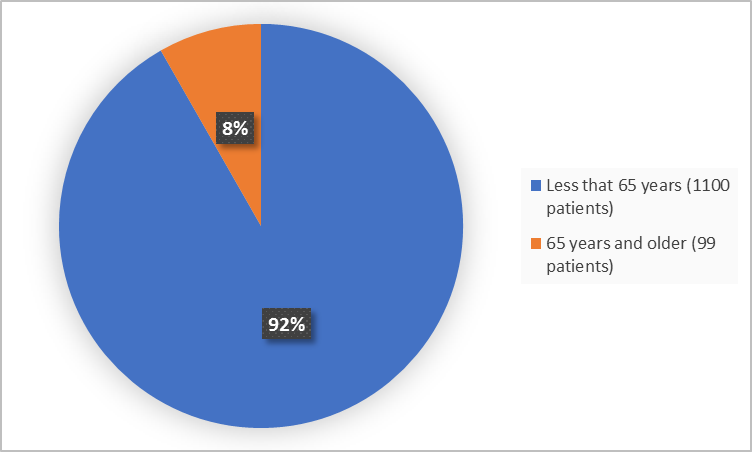
- Age: IBSRELA was similarly effective in patients younger and older than 65 years of age.
What are the possible side effects?
IBSRELA may cause serious dehydration in children. It should not be given to children younger than 6 years of age and avoided in children 6 to 12 years of age.
The most common side effects of IBSRELA are diarrhea, which sometimes may be severe, abdominal distension, excessive gas and dizziness.
Were there any differences in side effects among sex, race and age?
- Sex: The majority of patients in the trials were women. The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in different races.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved IBSRELA based primarily on evidence from two clinical trials Trial 1(NCT 02686138) and Trial 2(NCT 02621892) of 1199 patients 18 to 75 years of age with IBS-C. The trials were conducted at 214 sites in the United States.
Figure 1 summarizes how many men and women were in the clinical trials used to evaluate the benefit of IBSRELA.
Figure 1. Baseline Demographics by Sex (efficacy population)
Clinical Trial Data
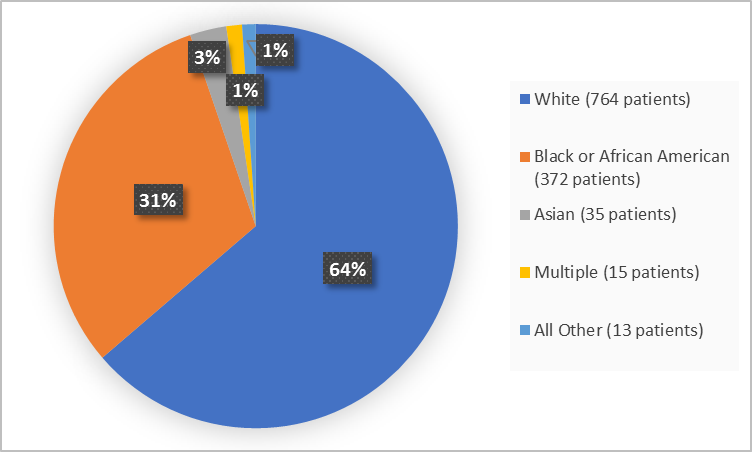
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trials used to evaluate the benefit of IBSRELA.
Figure 2. Baseline Demographics by Race (efficacy population)
Clinical Trial Data
Table 1. Demographics of Efficacy Trials by Race (efficacy population)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 764 | 64 |
| Black or African American | 372 | 31 |
| Asian | 35 | 3 |
| American Indian or Alaska Native | 3 | Less than 1 |
| Multiple | 15 | 1 |
| Other/Unknown | 10 | 1 |
Figure 3. Baseline Demographics by Age (efficacy population)
Clinical Trial Data
How were the trials designed?
There were two trials that evaluated the benefit and side effects of IBSRELA. In each trial, patients were randomly assigned to receive either IBSRELA or placebo two times daily for at least 12 weeks. Neither the patients nor the health care providers knew which treatment was being given. After these 12 weeks of treatment, patients in Trial 1 continued same trial design for an additional 14 weeks, and patients in Trial 2 entered a different trial design called Randomized Withdrawal for 4 weeks, where some patients continued to receive IBSRELA, and others were switched to placebo, in a blinded fashion.
In both trials, patients used diaries to record the degree of abdominal pain and stool frequency. The benefit of IBSRELA was assessed by improvements in abdominal pain and complete spontaneous bowel movements in comparison to placebo.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.






















.png)












No hay comentarios:
Publicar un comentario