
Combination of Immunotherapy Drugs Approved for Metastatic Colorectal Cancer
August 3, 2018, by NCI Staff
On July 10, the Food and Drug Administration (FDA) approved the combination of two immunotherapydrugs—ipilimumab (Yervoy) and nivolumab (Opdivo)—for the treatment of some patients with metastatic colorectal cancer who have been treated previously with standard chemotherapy drugs.
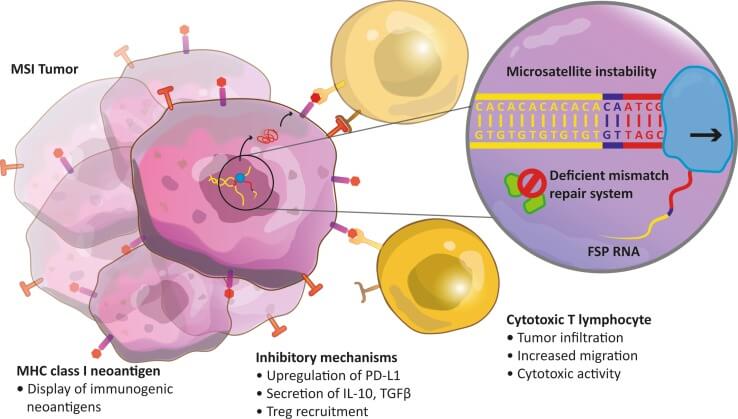
The accelerated approval covers patients 12 years of age and older whose tumors have one of two genetic features that prevent cells from repairing damaged DNA: mismatch repair deficiency (dMMR) or high microsatellite instability (MSI-H).
Tumors that have either of these genetic features tend to have high rates of DNA mutations. Some of these mutations may lead to the production of many abnormal antigens that can be targeted by immune cells.
Researchers have found that several immune checkpoint inhibitors are effective in patients with MSI-H or dMMR tumors, and this work has led to FDA approvals.
In 2017, FDA approved the checkpoint inhibitor pembrolizumab (Keytruda) for the treatment of MSI-H or dMMR tumors, regardless of where in the body the cancer started. This was the first FDA approval based solely on the presence of a genetic feature in a tumor.
The approval was a step forward for precision medicine, noted James Gulley, M.D., Ph.D., head of the immunotherapy section of NCI’s Center for Cancer Research.
A few months later, FDA approved nivolumab for patients with MSI-H or dMMR metastatic colorectal cancer that has progressed after chemotherapy.
The CheckMate-142 Study
The new FDA approval was based on results from the CheckMate-142 study, which was funded by Bristol-Myers Squibb, the manufacturer of ipilimumab and nivolumab.
The clinical trial is testing nivolumab alone and in combination with other anticancer drugs, including ipilimumab, in patients with dMMR or MSI-H metastatic colorectal cancer that had progressed after treatment with fluoropyrimidine- and oxaliplatin-containing chemotherapy or fluoropyrimidine- and irinotecan-containing chemotherapy.
Among 82 patients in the trial who received the combination immunotherapy, 3 had complete responses and 35 had partial responses, for an overall response rate of 46%. In addition, 89% of the responses lasted 6 months or longer, according to FDA.
The FDA statement noted that the overall response rate among a similar group of 58 patients who received nivolumab alone was 28%, with 67% of the responders having responses that lasted 6 months or more.
The most common side effects among the patients receiving ipilimumab and nivolumab included fatigue, diarrhea, fever, musculoskeletal pain, and abdominal pain. The combination immunotherapy had a “manageable safety profile,” the researchers wrote in a report on the CheckMate-142 study earlier this year.
Assessing the Various Treatment Options for Patients
Although CheckMate-142 did not include an arm testing pembrolizumab in the same patient population, Dr. Gulley noted that the response rates for both are similar (46% for the combination and 40% for pembrolizumab alone)—and higher than that for nivolumab alone (28%).
“This combination immunotherapy is an important additional option for patients,” he said, noting that pembrolizumab, as a single agent, may have fewer side effects than the two-drug combination.
“We don’t know if there are patients who would respond to nivolumab and ipilimumab who wouldn’t respond to pembrolizumab or vice versa,” Dr. Gulley continued.
He also noted that prospective studies would need to be done to see whether patients with dMMR or MSI-H metastatic colorectal cancer who didn’t respond to checkpoint inhibitors, like pembrolizumab, that block the protein PD-1 would respond to the addition of checkpoint inhibitors, like ipilimumab, that target the protein CTLA-4.





















.png)
.png)











No hay comentarios:
Publicar un comentario