
Spatial deconvolution of marker expression in whole heart subjected to ischemia-reperfusion
 Thought LeadersDr Gabriel BidauxResearch scientist, Principal InvestigatorInserm
Thought LeadersDr Gabriel BidauxResearch scientist, Principal InvestigatorInsermInterview with Dr. Gabriel Bidaux, conducted by Will Soutter MSc
Please give an overview of what happens to the heart during the ischemia-reperfusion sequence caused by myocardial infarction.
When a coronary artery is obstructed by atheroma, blood flow is depressed or even arrested. This causes hypoxia of cardiac cells associated with the deprivation of nutrients. In clinic, the first objective is to restore blood flow. However, this is associated with a burst in oxidation of cellular proteins and lipids. This oxidation enhances cell death and participates to the so-called reperfusion injury.
What problems have there been with understanding the ischemia-reperfusion process?
From the molecular scale to the organ scale, there are a lot of issues in performing this research. The first is the tissue heterogeneity: cardiomyocytes are not the only cell type forming the myocardium, although our strategy mostly aims to save them.
The second is the multi-scale nature of our object of research: molecular mechanisms can be studied at the single cell level. However, cells are organized into tissues and function as a whole organ. Understanding the molecular mechanisms occurring in cardiomyocytes upon ischemia-reperfusion does not guarantee the effective treatment of myocardial infarction, since the relation between the single cell level and the organ level is non-linear. This is probably why several worldwide clinical trials aiming to treat myocardial infarction have failed over the last few years.
Please describe your research into the ischemia-reperfusion mechanism.
As with many teams worldwide, we are studying the molecular mechanisms controlling cell death over ischemia-reperfusion. Although we are mainly focusing on what happens in the mitochondria of cardiomyocytes, we are also interested in the participation of immune cells at the onset of reperfusion.
Thanks to the high throughput methods coupled to systems biology, we have isolated expression markers from heart biopsies. However, we would like to understand where these markers are really expressed: at the necrotic core, in the periphery of the ischemic area, or in the healthy area.
How has the X-CLARITY Tissue Clearing System helped to quantify the living and necrotic tissue in your subjects?
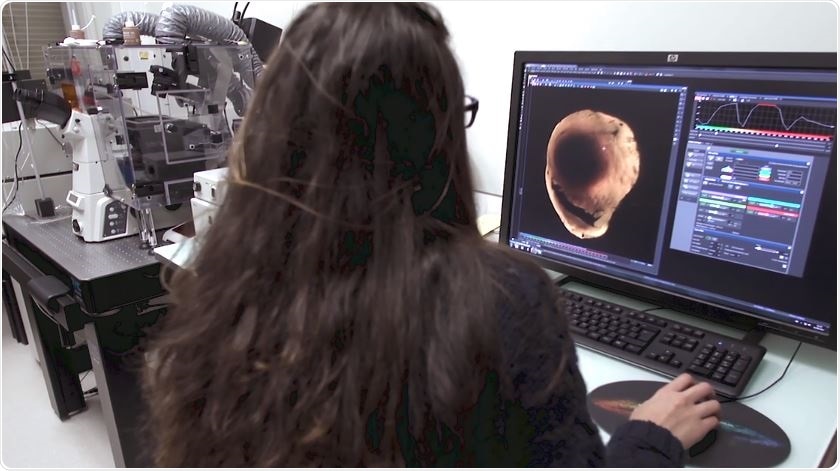
Thanks to the X-CLARITY Tissue Clearing System, we can now clear mouse hearts prior to imaging them with a fluorescence microscope. This enables the 3D rendering of fluorescent markers in the heart volume.
How do you label the necrotic and living tissue?
We first inject Unisperse Blue into the healthy area in order to increase the contrast between healthy and ischemic area.
We also tested different methods to label the damaged area. This is the most exciting part of our work.
What advantages are there to whole sample tissue clearing when compared to previous methodologies?
Previous methods are less spatially resolved and limited by the number of labels. Our research with clear hearts has been to detect the ischemic area, while being able to specifically label 2-3 markers of interest. Thanks to the revolution brought by light sheet microscope technology, we can now reconstruct the heart volume in 3D with unprecedented spatial resolution.
What does the future hold for your research? How do you hope your research will be applied in the future?
I hope that the chain-of-methods, including use of the X-CLARITY, will provide another quantitative tool for our colleagues studying ischemia-reperfusion. I also hope that these methods will provide us new valuable information in our lab’s aim to find out new molecular targets to improve cell survival after ischemia-reperfusion.
What future applications do you see for the X-CLARITY Tissue Clearing System within your group’s research?
We aim to perform spatial deconvolution of gene expression in healthy, peripheral, and ischemic areas. This “spatial” information will enrich our database of gene expression of mouse myocardium over the ischemia-reperfusion sequence, which we hope will provide us new molecular targets to heal myocardial infarction.
Where can readers find more information?
In our future research papers: https://www.researchgate.net/profile/Gabriel_Bidaux
About the X-CLARITY system: http://logosbio.com/x_clarity/x_clarity/features.php
About Gabriel Bidaux
 Dr. Gabriel Bidaux received his PhD from Lille University and defended his thesis in health and life sciences in Dr. Natalia Prevarskaya’s laboratory in 2006. He studied the involvement of the cold receptor TRPM8 in skin homeostasis, prostate cancer, and germ cell sensitivity to cold for 10 years.
Dr. Gabriel Bidaux received his PhD from Lille University and defended his thesis in health and life sciences in Dr. Natalia Prevarskaya’s laboratory in 2006. He studied the involvement of the cold receptor TRPM8 in skin homeostasis, prostate cancer, and germ cell sensitivity to cold for 10 years.From 2013 to 2015, he joined Dr. Laurent Héliot’s team in the Interdisciplinary Research Institute in Villeneuve d’Ascq as an associate researcher. He was introduced to biophysics and used state-of-the-art fluorescence microscopy and image analysis to study the dynamics of the recruitment of transcription factors on RNA polymerase II.
In 2015, he joined Pr. Michel Ovize’s cardioprotection team as a senior researcher in the CarMeN laboratory headed by Dr. Hubert Vidal. Since 2017, he holds a senior researcher position at INSERM and is developing his research group in Dr. Ovize’s team.
Dr. Bidaux’s research spans from developing new chains of methods (with Dr. Claire Crola Da Silva) and developing systems biology projects to study myocardial infarction with a focus on the mechanisms behind the cardioprotective effects of hypothermic conditioning.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.





















.png)












No hay comentarios:
Publicar un comentario