Study Shows Platelets Can Deliver Immunotherapy, Reduce Tumor Regrowth
March 3, 2017, by NCI Staff
A new study suggests that blood platelets engineered to deliver an immunotherapy drug may effectively eliminate cancer cells missed by surgery and prevent them from forming new tumors.
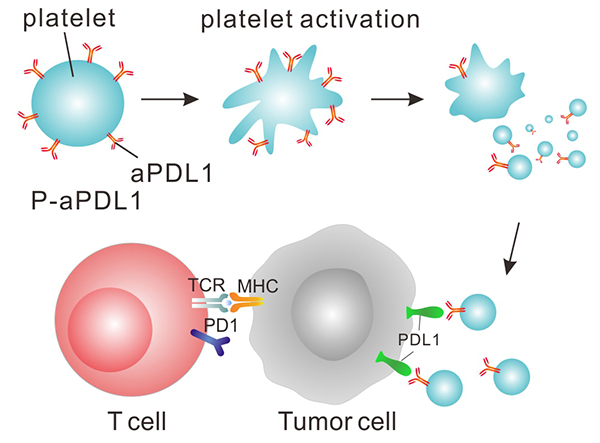
In the study, the researchers chemically linked platelets—which normally help form blood clots and heal wounds—to an immune checkpoint inhibitor, a form of immunotherapy that releases the brakes on the anticancer immune response.
Using mouse models of melanoma and breast cancer from which the majority of the tumor had been surgically removed, they found that mice treated with the engineered platelets had reduced tumor regrowth and metastasis and lived longer than mice treated with normal platelets or the checkpoint inhibitor alone.
This targeted approach, tested only in mice so far, could potentially produce fewer side effects than traditional immunotherapy, the study team believes.
“I like this approach,” said James Gulley, M.D., Ph.D., chief of the Genitourinary Malignancies Branch and head of the Immunotherapy Group in NCI’s Center for Cancer Research, who was not involved in the study. “It could add an extra insurance policy for patients whose tumors are potentially curable with surgery.”
The study, led by Zhen Gu, Ph.D., of the University of North Carolina at Chapel Hill and North Carolina State University, was published online January 23 in Nature Biomedical Engineering.
Creating a Targeted Drug Carrier
Many patients with solid tumors that have not spread undergo surgery to have their tumor removed. However, it can be challenging to remove the entire tumor, and some cancer cells may remain after surgery. These residual cancer cells have the potential to regrow at the site of the original tumor or spread to form new tumors in other organs.
Dr. Gu and his colleagues wondered whether immunotherapy drugs could be targeted to the surgery site as an adjuvant therapy to help wipe out any remaining cancer cells. They chose a checkpoint inhibitor that blocks PD-L1 protein on cancer cells, enabling immune cells to attack cancer cells.
To deliver the therapy, they sought the help of platelets because these small cell fragments accumulate in wounds and can interact with metastatic cancer cells circulating in the bloodstream. In addition, when platelets become activated at the wound site, they release chemicals to augment the local immune response helping to repair the wound.
The research team chemically linked a PD-L1 inhibitor to platelets they isolated from mice. They found that activation of the engineered platelets in the laboratory caused them to release the inhibitor along with the expected immune system-stimulating chemicals.
Next, they surgically removed approximately 99% of tumors in mice with melanoma, then injected the mice with inhibitor alone or the engineered platelets. Using fluorescent tags, the researchers found that, 2 hours after injection, the engineered platelets accumulated at the surgery wound site, whereas the inhibitor alone did not.
And, compared with mice treated with surgery only, mice treated with surgery and the engineered platelets had higher levels of immune system-stimulating chemicals and cancer-fighting immune cells at the wound site. However, these chemicals were not elevated in the bloodstream of mice treated with engineered platelets, indicating that the immune system activation was localized to the wound site.
Most importantly, compared to mice treated with normal platelets or the inhibitor alone, mice treated with the engineered platelets had the least amount of tumor regrowth after surgery, and they lived substantially longer.
To test whether the engineered platelets could prevent the formation of metastatic tumors, the researchers injected melanoma cells into the bloodstream of mice from whom tumors had been removed surgically. At the same time, they injected the mice with normal platelets, PD-L1 inhibitor alone, or engineered platelets.
After tracking the mice for about seven weeks, they found that only mice treated with the engineered platelets had reduced numbers of metastatic lung tumors as well as reduced growth of the original tumor. In addition, whereas none of the mice treated with normal platelets or inhibitor alone survived past 40 days, about half of the mice treated with the engineered platelets were still alive at that time.
The researchers observed similar results when they treated mice bearing an aggressive type of breast cancer with the engineered platelets.
Maximizing Potential
A human PD-L1 inhibitor, atezolizumab (Tecentriq®), and several other checkpoint inhibitors have been approved by the Food and Drug Administration to treat patients with various types of advanced cancer. These drugs have been found to produce long-lasting responses in a small percentage of patients.
Like Dr. Gu and his colleagues’ engineered platelet approach, clinical researchers are also exploring the use of immune checkpoint inhibitors as an adjuvant therapy after surgery, noted Morteza Mahmoudi, Ph.D., and Omid Farokhzad, M.D., of Boston’s Brigham and Women's Hospital in an accompanying editorial.
The goal of using immunotherapy as an adjuvant therapy “is to diminish the risk of tumor recurrence from microscopic tumors in surgical margins and of micrometastases seeded by circulating tumor cells,” they wrote.
Particularly in the case of patients for whom surgery may be curative, it’s important that any post-surgical therapy be safe and not lessen patients’ quality of life, Dr. Gulley explained. “The ideal adjuvant therapy would be something that could improve outcomes but not cause substantial symptoms for the patient,” he said.
Although checkpoint inhibitors are generally well tolerated, in rare cases they can send the patient’s immune system into overdrive, leading to serious damage of healthy cells. For example, some inhibitors have caused autoimmune disorders, inflammation, and even type 1 diabetes, Dr. Gu said.
“We are trying a local, or targeted, delivery approach to prevent these kinds of side effects,” he added.
Although the current study aimed to test the efficacy of the engineered platelets, the researchers plan to conduct further studies to determine if these platelets cause fewer side effects than the PD-L1 inhibitor on its own.
And Dr. Gu thinks the team’s approach is just the beginning.
“We call it a platform technology. We used platelets to deliver a PD-L1 inhibitor, but platelets could certainly be used as a vehicle or carrier to transport other therapeutics, including other antibodies, small molecules, or chemotherapeutics.”
As far as the potential of the engineered platelets as a treatment, Dr. Gulley said, “it’s unclear how this approach would translate in patients. But I think it certainly raises the hypothesis that one could improve upon current checkpoint inhibitor strategies by additional targeting to the tumor.” It would be interesting to determine if the engineered platelets prevent the spread of cancer in mouse models that are more representative of metastatic cancer, he added.
In a similar type of approach, Dr. Gulley and his NCI colleagues are exploring the clinical potential of a tumor-targeted immunocytokine, a molecule that recruits cancer-killing immune cells, as an alternative to treatment with the cytokine alone.





















.png)












No hay comentarios:
Publicar un comentario