
NIH, Fogarty collaborate on COVID-19 rapid response
March / April 2020 | Volume 19, Number 2

Courtesy of NIBIB/NIH
NIH scientists are working on a wide range of COVID-19
activities, including a study to determine the presence of
COVID-19 antibodies in a sampling of the U.S. population.
activities, including a study to determine the presence of
COVID-19 antibodies in a sampling of the U.S. population.
While many research projects have been stopped or delayed, NIH scientists are working flat out on all aspects of the novel coronavirus, including developing diagnostics, studying potential treatments and producing an effective vaccine. NIH leadership has established 10 cross-cutting work groups to focus and coordinate efforts. Fogarty staff are participating in a number of them to ensure the global health perspective is represented.
To fast-track progress, NIH has announced a public-private partnership that will develop an international strategy for a coordinated research response. The planned program - Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) - will develop a collaborative framework for prioritizing vaccine and drug candidates, streamlining clinical trials, coordinating regulatory processes and/or leveraging assets among all partners to rapidly respond to the COVID-19 and future pandemics.
“We need to bring the full power of the biomedical research enterprise to bear on this crisis,” said NIH Director Dr. Francis S. Collins. “Now is the time to come together with unassailable objectivity to swiftly advance the development of the most promising vaccine and therapeutic candidates that can help end the COVID-19 global pandemic.”
Coordinated by the Foundation for the NIH, ACTIV government and industry partners will provide infrastructure, subject matter expertise and/or funding to identify, prioritize and facilitate the entry of some of the most promising candidates into clinical trials. Industry partners also will make available certain prioritized compounds, some of which have already cleared various phases of development, and associated data to support research related to COVID-19.
NIH has posted treatment guidelines for COVID-19 on its website. Developed by a panel of NIH leaders, physicians, statisticians and other experts, the guidelines provide clinical data on therapies, ongoing clinical trials and known interactions with other drugs. Recommendations also include best practices for managing patients at different stages of infection. The information will be updated as new data are published.
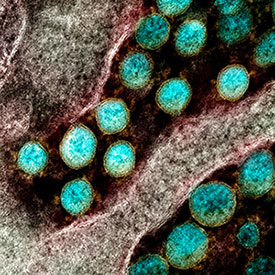
Image courtesy of NIAID
Novel Coronavirus SARS-CoV-2
The National Institute of Allergy and Infectious Diseases (NIAID), which is leading the NIH response to COVID-19, has also developed a vaccine candidate, opened a number of clinical trials to study possible therapeutics and published timely research findings. The Institute is supporting a randomized controlled trial of remdesivir in hospitalized adults diagnosed with COVID-19, and is exploring other broad-spectrum antiviral compounds. NIAID plans to evaluate possible treatments, such as the HIV medication Kaletra - also known as lopinavir and ritonavir - as well as interferon-beta. In addition, NIAID is investigating several antibody-based therapeutics, which prevent the virus from infecting and entering cells. NIAID also collaborated with biotech firm Moderna, Inc., to develop a vaccine against COVID-19. Phase I clinical trials are underway.
An NIAID study has validated decontamination methods for re-use of N95 respirators, which are designed for single-use and worn by healthcare providers. Finally, NIAID has begun a large-scale study to find out how many adults who have not been diagnosed with COVID-19 carry antibodies to the virus.
Fogarty staff have also swung into action. With Fogarty Fellow Angela Spencer one of 7,000 Americans stranded in Peru, program officer Kevin Bialy worked his connections at the State Department to help Spencer be repatriated. The Oregon Health and Science University Ph.D. candidate is studying elements of community-based cysticercosis control with collaborators at Cayetano University’s Center for Global Health in the remote city of Tumbes. Although disappointed her field research was cut short, Spencer said she was encouraged by the way people stepped forward to help others and was reminded “there are many good people who use their time and talents to make the world a better place.” Spencer said she is more committed than ever to a career in global health because, “diseases don’t stop at borders, and we can learn so much from one another.”
Fogarty also worked diplomatic relationships to ensure reagents being donated by Johns Hopkins University would be delivered on a State Department flight to Africa, where scientists needed them to conduct coronavirus studies.
When the outbreak began, Fogarty’s team of infectious disease modelers immediately began analyzing data from China, led by Dr. Cécile Viboud. In quick succession, the team published several papers on the outbreak’s spread in China and the effect of travel restrictions . In the pipeline are papers including a study of the response in South Korea, the difficulty of gathering reliable mortality figures during an outbreak and seroprevalence of antibodies among contacts of COVID-19 patients.

Photo courtesy of Javed Muhammad
Fogarty has supported biosafety training in Pakistan since
2014. Lessons learned are being applied to the country’s
COVID-19 response.
2014. Lessons learned are being applied to the country’s
COVID-19 response.
In Pakistan, Fogarty biosafety trainees were prepared to begin COVID-19 testing and research, having mastered topics such as control of air flow in labs, proper procedures for personal protective equipment, decontamination practices and other aspects of biosecurity. The training - led by Fogarty’s Dr. Zeba Rasmussen - began in 2014 in partnership with the Pakistan Biological Safety Association .
In typical Fogarty fashion, many of the program’s trainees have now become the trainers. As her province geared up preparations for COVID-19, program participant Dr. Hafsah Muhammad of Khyber Medical University began training health care workers and lab personnel, and helped Dr. Yasar Yousafzai, Director, take the lead to set up the main Provincial Public Health Reference lab, to provide testing. “Indeed everything we learned in our PBSA/Fogarty training is now being used practically in the COVID-19 outbreak,” she said.
COVID-19 makes more urgent the need for quality control on the global supply of diagnostics, medicines and vaccines, according to Dr. Joel Breman, Fogarty senior scientist emeritus and co-author of an article published in The Lancet Global Health . “The COVID-19 pandemic threatens a global surge in substandard and falsified medical products, not just for those directly related to COVID-19,” the article stated. “Many products essential for COVID-19 treatment and prevention are at risk - including face masks, hand sanitizer and diagnostic tests - and false claims have been made for prevention and treatment.”
More Information
- Coronavirus news, funding and resources for global health researchers from Fogarty
- NIAID strategic plan details COVID-19 research priorities
NIAID/NIH news, April 23, 2020 - NIH to launch public-private partnership [Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)] to speed COVID-19 vaccine and treatment options
NIH news, April 17, 2020 - Coronavirus Disease 2019 (COVID-19) Treatment Guidelines
- Expert US panel develops NIH treatment guidelines for COVID-19
NIAID/NIH news, April 21, 2020 - Developing therapeutics and vaccines for coronaviruses from NIAID/NIH
- NIH study validates decontamination methods for re-use of N95 respirators
NIAID/NIH news, April 15, 2020 - NIH begins study to quantify undetected cases of coronavirus infection
NIAID/NIH news, April 10, 2020 - Comment: Impact of contact tracing on SARS-CoV-2 transmission , authored by Fogarty scientists Drs. Kaiyuan Sun and Cecile Viboud
The Lancet Infectious Diseases, April 27, 2020 - Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study [Open access] , authored by Fogarty scientists
The Lancet Digital Health, February 20, 2020 - The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak , co-authored by Fogarty's Dr Cecile Viboud
Science, March 6, 2020 - Pakistan Biological Safety Association
- COVID-19 and risks to the supply and quality of tests, drugs, and vaccines
The Lancet Global Health, April 9, 2020
To view Adobe PDF files, download current, free accessible plug-ins from Adobe's website .





















.png)












No hay comentarios:
Publicar un comentario