A new DRUG TRIALS SNAPSHOT is now available.

Drug Trials Snapshot: PEMAZYRE
PEMAZYRE is a drug used for treatment of adults with bile duct cancer (cholangiocarcinoma) that has spread to other parts of the body (metastatic) or cannot not be removed by surgery. It should be used in patients who have been previously treated with chemotherapy and whose cancer has a certain type of abnormality in the FGFR2 gene.
PEMAZYRE is a tablet taken by mouth once a day for 14 days followed by 7 days off treatment to complete a 21-day treatment cycle.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
PEMAZYRE (pemigatinib)
(pemah zeer)
Incyte Corporation
Approval date: April 17, 2020
(pemah zeer)
Incyte Corporation
Approval date: April 17, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
PEMAZYRE is a drug used for treatment of adults with bile duct cancer (cholangiocarcinoma) that has spread to other parts of the body (metastatic) or cannot not be removed by surgery. It should be used in patients who have been previously treated with chemotherapy and whose cancer has a certain type of abnormality in the FGFR2 gene.
How is this drug used?
PEMAZYRE is a tablet taken by mouth once a day for 14 days followed by 7 days off treatment to complete a 21-day treatment cycle.
What are the benefits of this drug?
In the trial, 38 of 107 patients (36%) treated with PEMAZYRE achieved partial or complete shrinkage of the tumor (overall response rate). Of these patients, 63% had a cancer shrinkage lasting 6 months or longer and 18% had it lasting 12 months or longer.
PEMAZYRE was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: PEMAZYRE worked similarly in men and women.
- Race: The majority of patients were White, therefore difference in how well the drug worked between races could not be determined.
- Age: PEMAZYRE worked similarly in patients above and below 65 years of age.
What are the possible side effects?
PEMAZYRE may cause serious side effects including detachment of retina (inner layer of the eye), increased phosphate level in the blood, and harm to unborn baby.
The most common side effects of PEMAZYRE are increased phosphate level in the blood, hair loss, diarrhea, nail changes, fatigue, change of taste sense, and nausea.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients were White, therefore differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients above and below 65 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved PEMAZYRE based on evidence from one clinical trial (NCT02924376) of 146 patients with previously treated, locally advanced or metastatic bile duct cancer. The trial was conducted at 67 sites in the United States, Europe, and Asia.
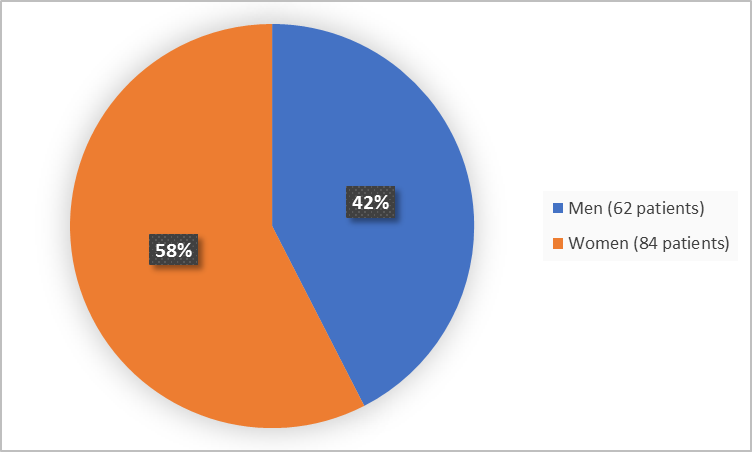
Figure 1 summarizes by sex how many patients were in the clinical trial.
Figure 1. Baseline Demographics by Sex (safety population)
Adapted from FDA Review
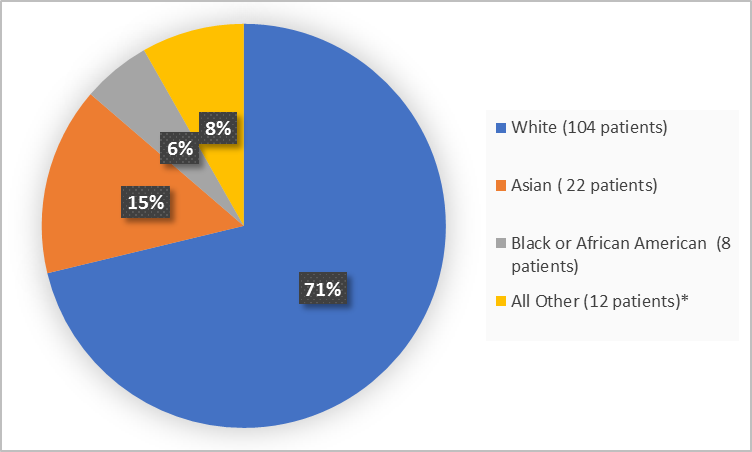
Figure 2 summarizes patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race (safety population)
*includes American Indian or Alaska Native, Other and Missing
Adapted from FDA Review
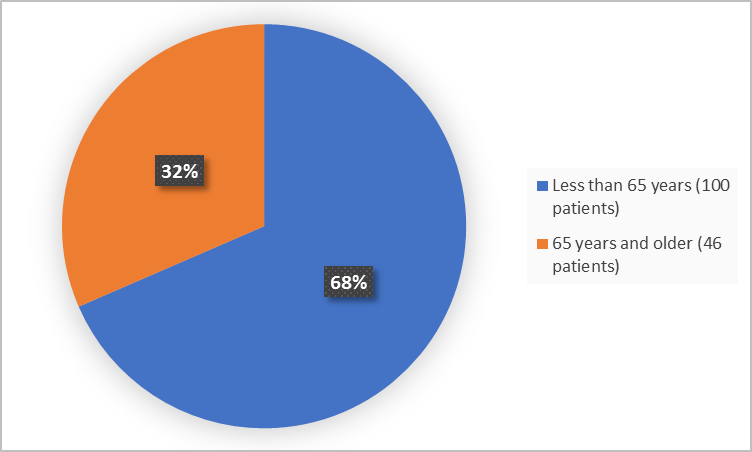
Figure 3 summarizes patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age (safety population)
Adapted from FDA Review
Figure 4 summarizes patients by ethnicity in the clinical trial.
Figure 4. Baseline Demographics by Ethnicity (safety population)
Adapted from FDA Review
How were the trials designed?
There was one trial that provided data for PEMAZYRE approval. The trial enrolled adult patients with bile duct cancer who had been treated previously with chemotherapy for their advanced cancer and whose tumors had a certain type of abnormality in the FGFR2 gene.
Patients received PEMAZYRE once daily by mouth for 14 consecutive days followed by 7 days off therapy. This 21-day cycle was administered until disease progression or the side effects became too toxic.
The trial measured the percentage of patients who achieved partial or complete shrinkage of their cancer and how long that shrinkage lasted (duration of response or DoR).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.






















.png)












No hay comentarios:
Publicar un comentario