A new DRUG TRIALS SNAPSHOT is now available.

Drug Trial Snapshot: TRODELVY
TRODELVY is a drug for the treatment of adults with triple-negative breast cancer that has spread to other parts of the body (metastatic). It should be used in patients who have received at least two prior therapies for their metastatic disease.
Triple-negative breast cancer is a type of breast cancer that tests negative for estrogen receptors, progesterone receptors and human epidermal growth factor receptor 2 (HER2) protein.
TRODELVY is an injection. It is given directly into the vein (intravenous infusion) by a healthcare provider once weekly on Days 1 and 8 of 21-day treatment cycles.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
TRODELVY (sacituzumab govitecan-hziy)
(troh-DELL-vee)
Immunomedics, Inc.
Approval date: April 22, 2020
(troh-DELL-vee)
Immunomedics, Inc.
Approval date: April 22, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TRODELVY is a drug for the treatment of adults with triple-negative breast cancer that has spread to other parts of the body (metastatic). It should be used in patients who have received at least two prior therapies for their metastatic disease.
Triple-negative breast cancer is a type of breast cancer that tests negative for estrogen receptors, progesterone receptors and human epidermal growth factor receptor 2 (HER2) protein.
How is this drug used?
TRODELVY is an injection. It is given directly into the vein (intravenous infusion) by a healthcare provider once weekly on Days 1 and 8 of 21-day treatment cycles.
What are the benefits of this drug?
The trial measured the percent of patients whose tumors completely or partially shrank after treatment (objective response rate). In the trial, approximately 30 percent of 108 patients taking TRODELVY experienced complete or partial shrinkage of their tumors that lasted about 8 months.
TRODELVY was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
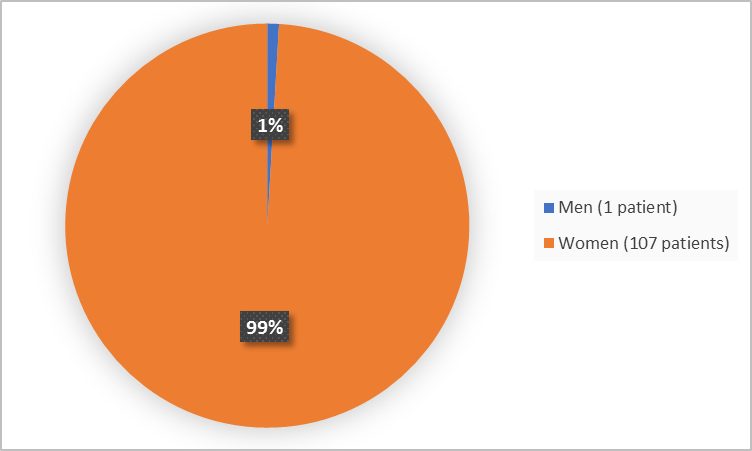
- Sex: Almost all included patients were women, therefore sex differences could not be determined.
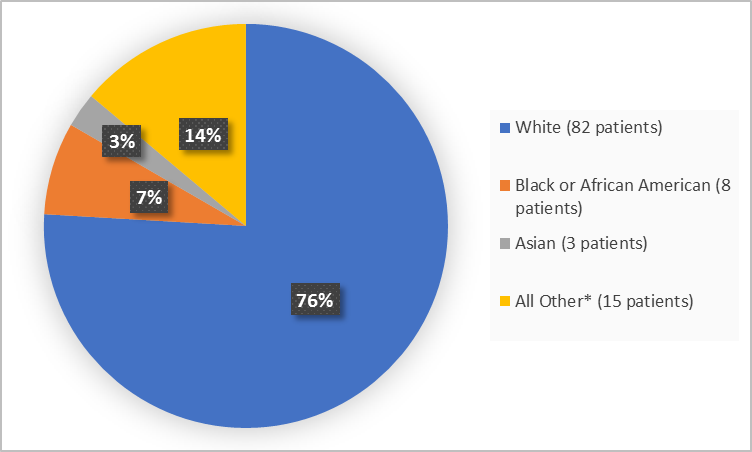
- Race: The majority of patients were White, therefore race differences could not be determined.
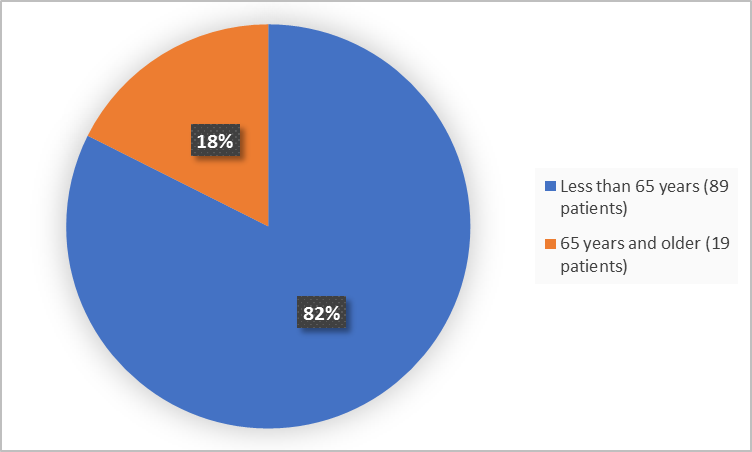
- Age: TRODELVY worked similarly in patients above and below 65 years of age.
What are the possible side effects?
TRODELVY may cause serious side effects including low white cell blood count (neutropenia), severe diarrhea, life-threatening allergic reactions, nausea and vomiting.
The most common side effects of TRODELVY are nausea, neutropenia, diarrhea, fatigue, anemia, vomiting, hair loss, constipation, rash, decreased appetite, and abdominal pain.
Were there any differences in side effects among sex, race and age?
- Sex: Almost all included patients were women; therefore, sex differences could not be determined.
- Race: The majority of patients were White; therefore, race differences could not be determined.
- Age: The occurrence of overall side effects was similar in patients above and below 65 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved TRODELVY based on evidence from one clinical trial (NCT01631552) of 108 patients with triple-negative metastatic breast cancer. The trial was conducted at 10 sites in the United States.
Figure 1 summarizes by sex how many patients were in the clinical trial.
Figure 1. Baseline Demographics by Sex
Adapted from FDA Review
Figure 2 summarizes patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
*includes American Indian or Alaska Native and Other
Adapted from FDA Review
Figure 3 summarizes patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age
Adapted from FDA Review
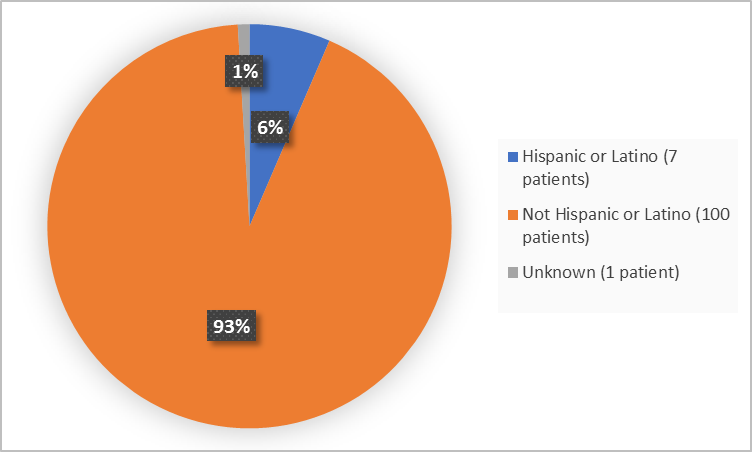
Figure 4 summarizes patients by ethnicity in the clinical trial
Figure 4. Baseline Demographics by Ethnicity
Adapted from FDA Review
How were the trials designed?
There was one trial that provided data for TRODELVY approval. The trial enrolled adult patients with metastatic triple-negative breast cancer (mTNBC) who had received at least two prior treatments for their metastatic disease.
Patients received TRODELVY intravenously on Days 1 and 8 of a 21-day treatment cycle. The treatment continued until the disease progressed or the side effects became too toxic.
The trial measured the percent of patients whose tumors completely or partially shrank during the treatment and how long that shrinkage lasted (duration of response).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.





















.png)












No hay comentarios:
Publicar un comentario